Product Details
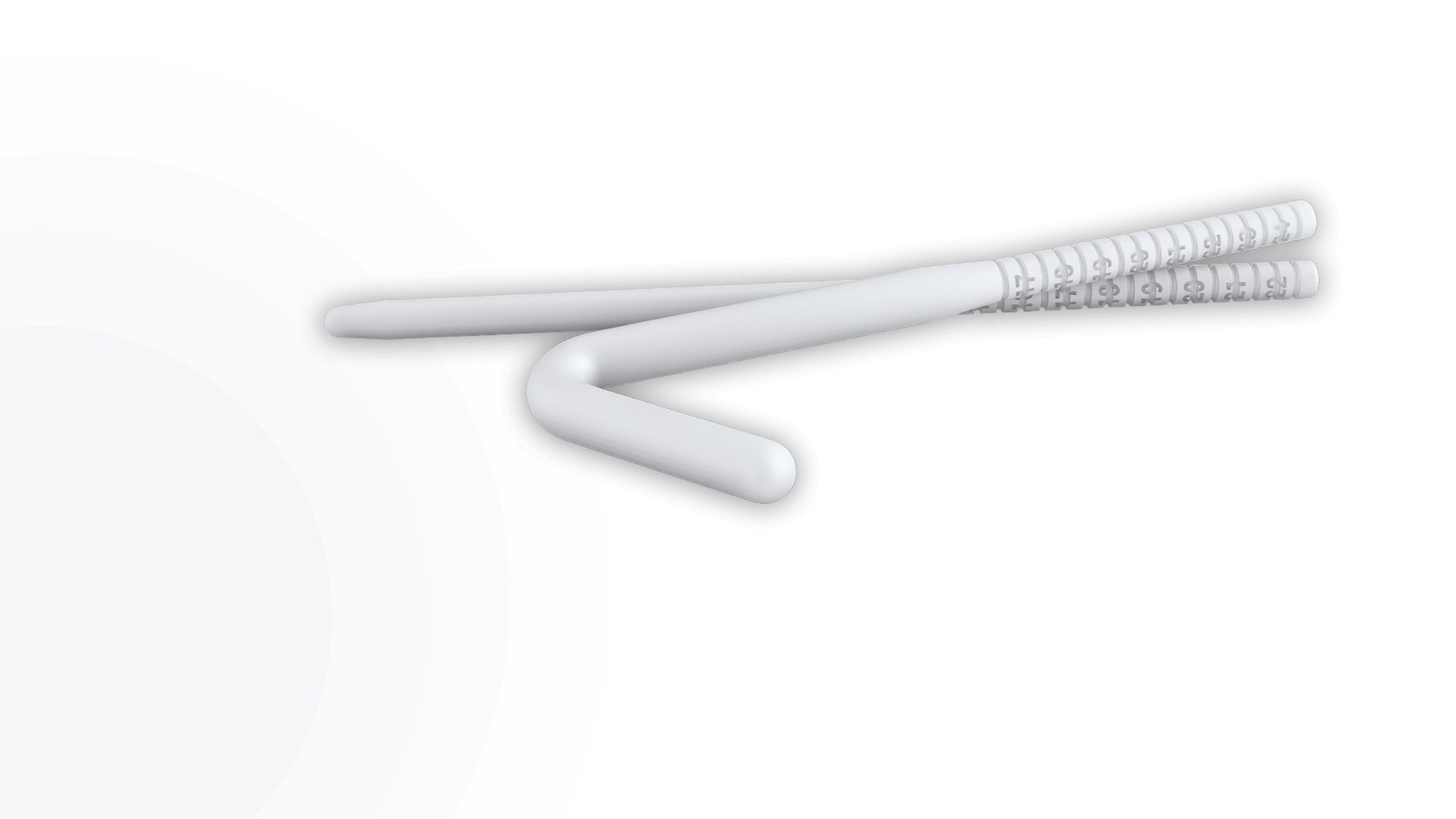
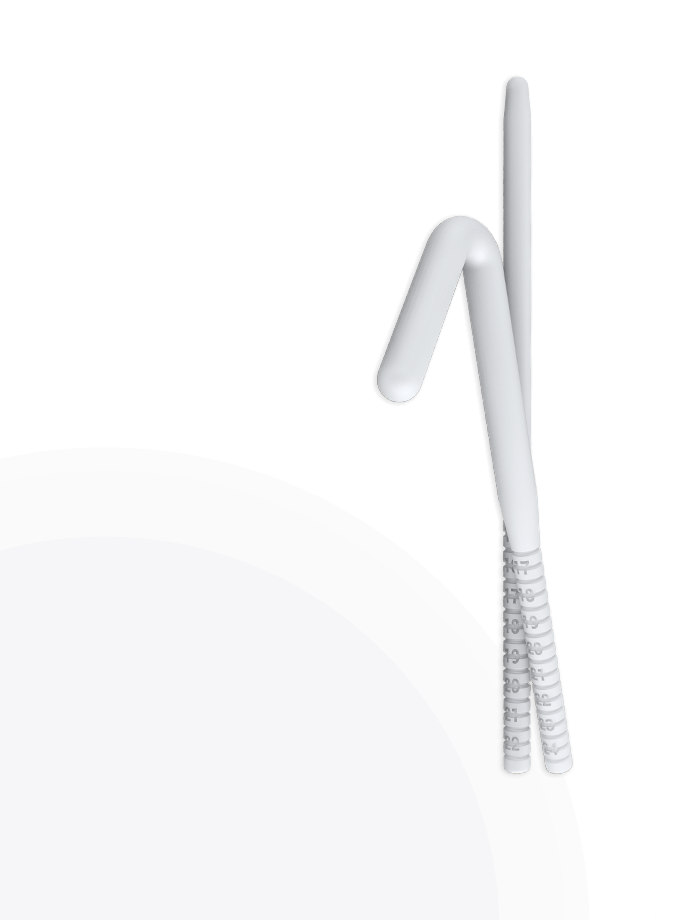
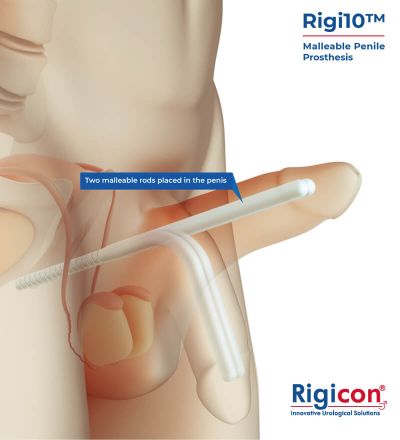
Rigi10™, Rigicon’s new malleable penile prosthesis, offers physicians an option that is easy to size, extend, and implant.
It is engineered to provide patients with a durable treatment option that ensures the required rigidity, comfort, and discretion.
Rigi10™ represents a unique combination of Rigicon’s expertise in men’s health and the latest advancements in medical technology.
| Key Features | Rigi10™ |
|---|---|
|
Bending Angle |
Up to 135° (Flexible Rod Technology™) |
|
Maximum Diameter |
14 mm |
|
Available Diameters |
9, 10, 11, 12, 13, 14* mm |
|
Surface Coating |
HydroShield™ Hydrophilic Coating |
Rigi10™ is designed for discretion—no one will know you have an implant unless you choose to share. Its high bending capacity and minimal spring-back provide dependable and easily concealable results.
To effectively address the unique anatomical requirements of patients, the Rigi10™ Malleable Penile Prosthesis offers a wider range of options with six different diameters—9, 10, 11, 12, 13, and 14* mm—and lengths of 23 cm for the 9 and 10 mm sizes, and 25 cm for the 11 to 14 mm sizes. Since there’s no sure way to determine penile anatomy size before surgery, these diverse sizing options enable surgeons to tailor the prosthesis for an optimal fit to each patient.
Rigi10™ features a HydroShield™ hydrophilic coating on both the device surface and rear tip extenders, offering surgeons the flexibility to select their preferred dipping solution while potentially promoting easier device implantation2.

To effectively address the unique anatomical requirements of patients, the Rigi10™ Malleable Penile Prosthesis offers a wider range of options with six different diameters—9, 10, 11, 12, 13, and 14* mm—and lengths of 23 cm for the 9 and 10 mm sizes, and 25 cm for the 11 to 14 mm sizes. Since there’s no sure way to determine penile anatomy size before surgery, these diverse sizing options enable surgeons to tailor the prosthesis for an optimal fit to each patient.
Rigi10™ features a HydroShield™ hydrophilic coating on both the device surface and rear tip extenders, offering surgeons the flexibility to select their preferred dipping solution while potentially promoting easier device implantation2.
Rigi10™ is designed for discretion—no one will know you have an implant unless you choose to share. Its high bending capacity and minimal spring-back provide dependable and easily concealable results.


Soft-to-touch tip
Soft-to-touch distal tip gives the patient a comfortable feel during daily activities.

Angled tip

HydroShield™ Coating

HydroShield™ Coating
Rigidity
Rigidity
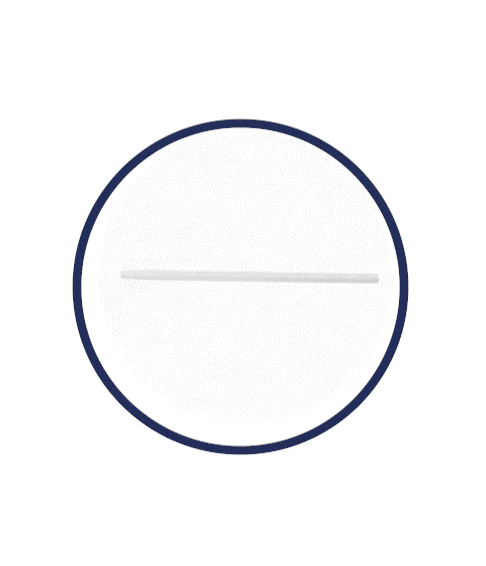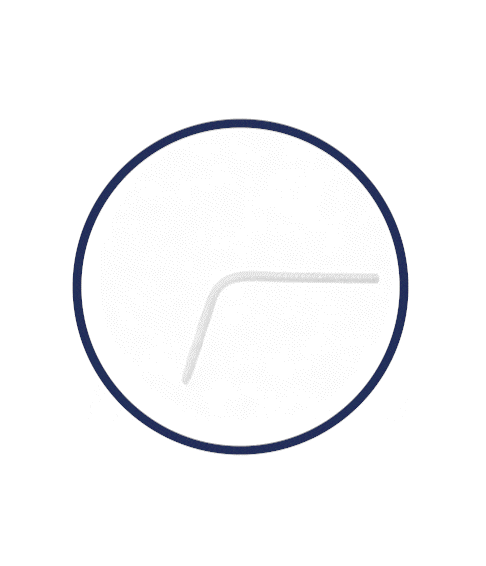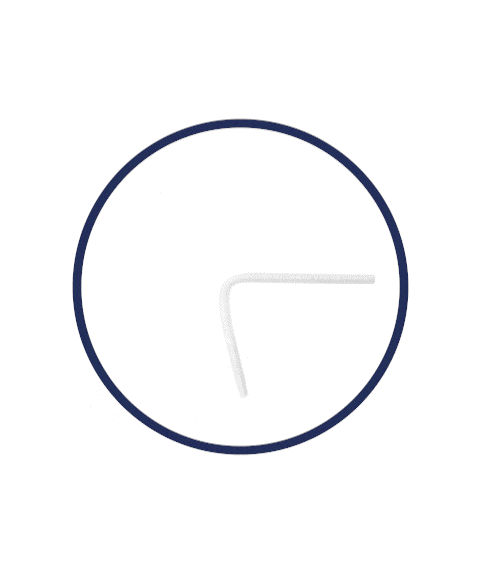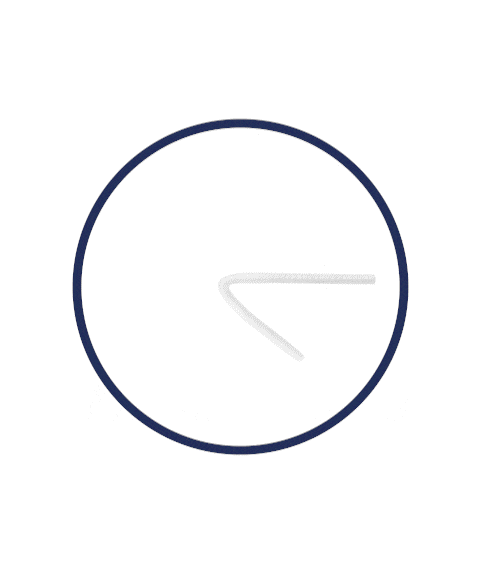
Flexible Rod Technology™

Flexible Rod Technology™

SecureFit™ RTE

SecureFit™ RTE
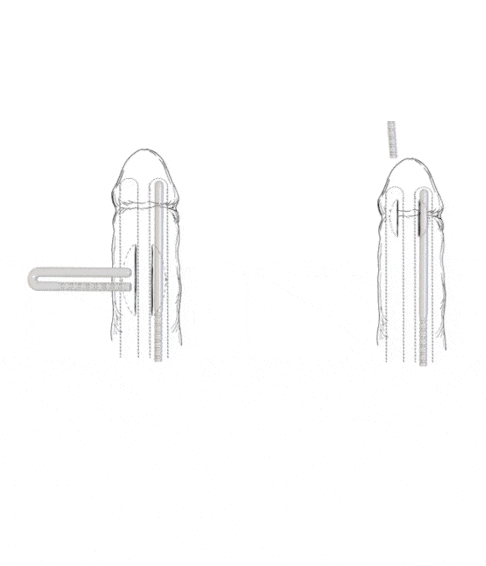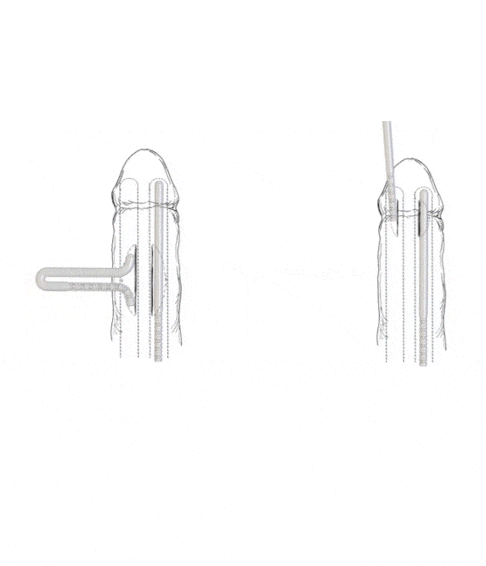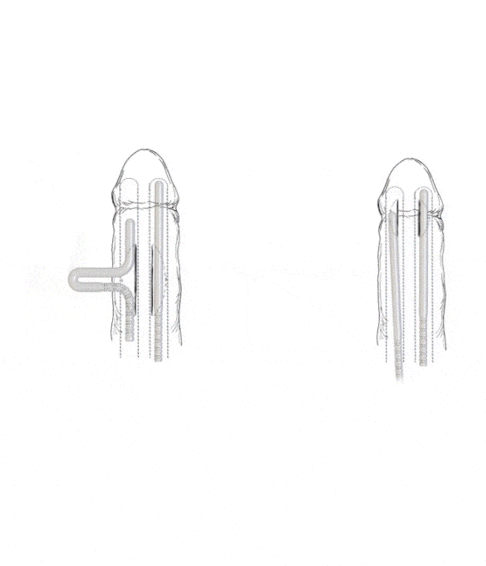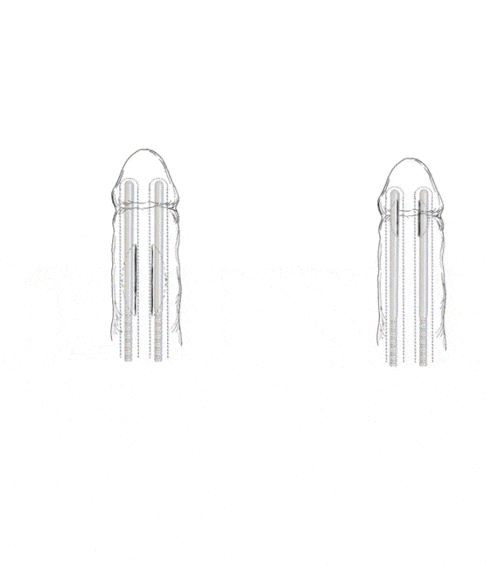
- Subcoronal placement of the Rigi10™
- Rigi10™ Introduction
- Rigi10™ Features and Benefits at a Glance
- Dr. Bhavik Shah Impressions on Malleable Penile Prosthesis and Rigi10™
- Rigicon Talks – Session 1 – Top 10 Questions Answered about Penile Implants
- Rigicon Talks – Session 3 – Myths and Facts About Penile Implants
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
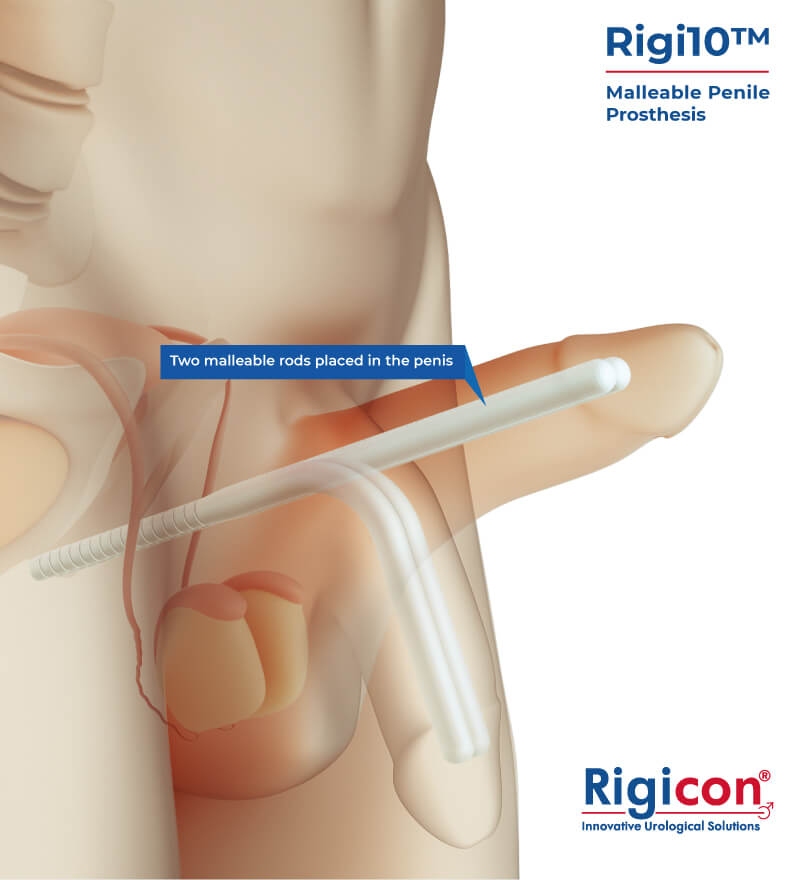
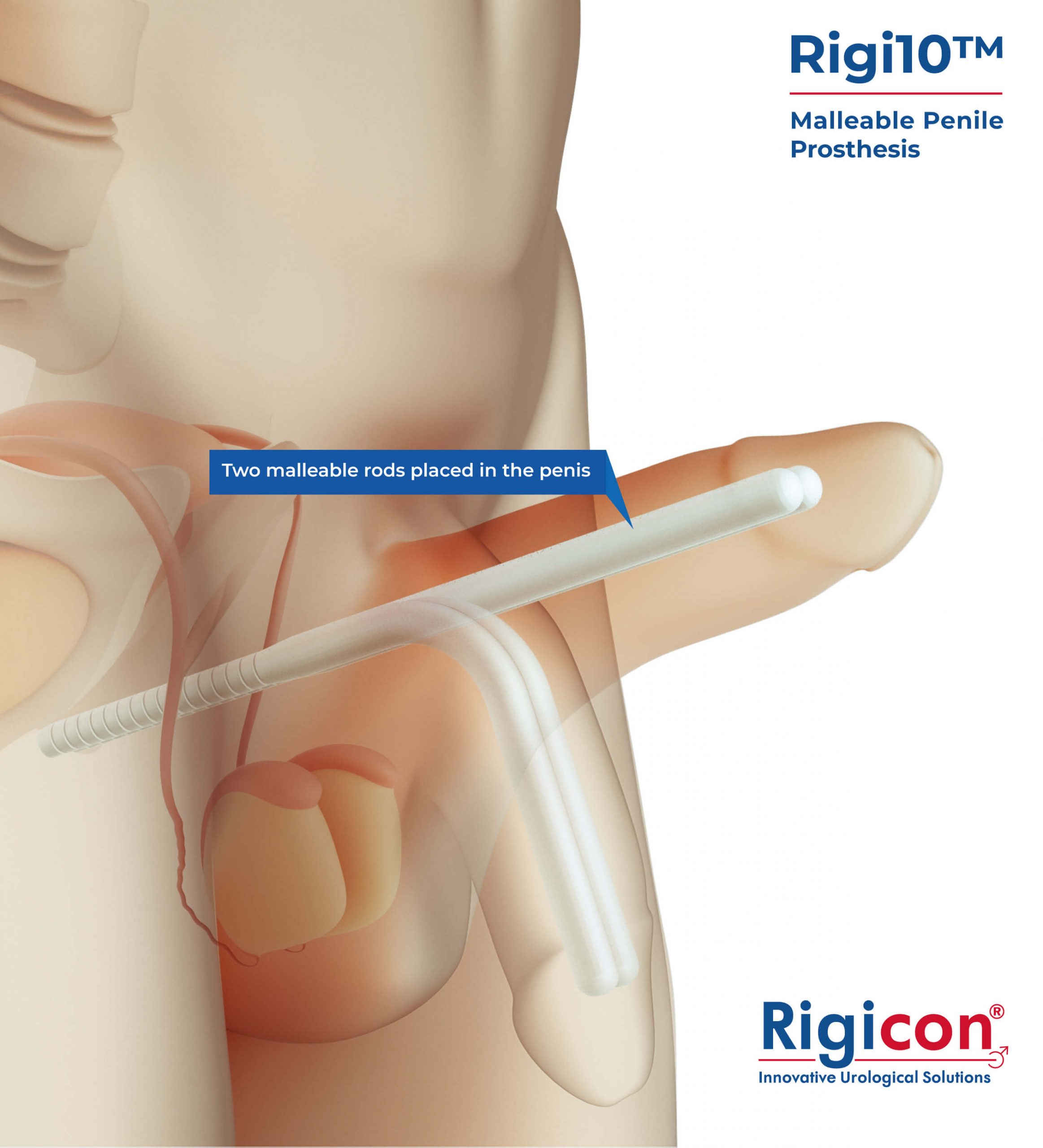
The Rigi10™ offers a potential long-term solution for men seeking consistent erectile function. This malleable penile prosthesis consists of two bendable rods surgically placed within the penis, providing a reliable and straightforward mechanism for achieving and maintaining rigidity during sexual activity. The prosthesis is designed to allow for a natural appearance both when in use and at rest, giving users the ability to manually adjust the position according to their needs.
Engineered for ease of use and durability, the Rigi10™ aims to provide a comfortable experience and help restore confidence and control in intimate moments.
The Rigi10™ offers a potential long-term solution for men seeking consistent erectile function. This malleable penile prosthesis consists of two bendable rods surgically placed within the penis, providing a reliable and straightforward mechanism for achieving and maintaining rigidity during sexual activity. The prosthesis is designed to allow for a natural appearance both when in use and at rest, giving users the ability to manually adjust the position according to their needs.
Engineered for ease of use and durability, the Rigi10™ aims to provide a comfortable experience and help restore confidence and control in intimate moments.
Product Code | Diameter | Length |
|---|---|---|
| RG1009H | 09 mm | 23 cm |
| RG1010H | 10 mm | 23 cm |
| RG1011H | 11 mm | 25 cm |
| RG1012H | 12 mm | 25 cm |
| RG1013H | 13 mm | 25 cm |
| RG1014H* | 14 mm | 25 cm |
What is erectile dysfunction (ED)?
Previously known as impotence, erectile dysfunction (ED) refers to the inability to achieve and maintain an erection sufficient for sexual intercourse. It is unrelated to sexual desire, libido, ejaculation, or pleasure. ED is solely concerned with the ability to achieve adequate penile stiffness for engaging in sexual activity.
What is a penile prosthesis?
A penile prosthesis is a sterile device implanted in men to provide sufficient penile rigidity for sexual penetration. There are two types:
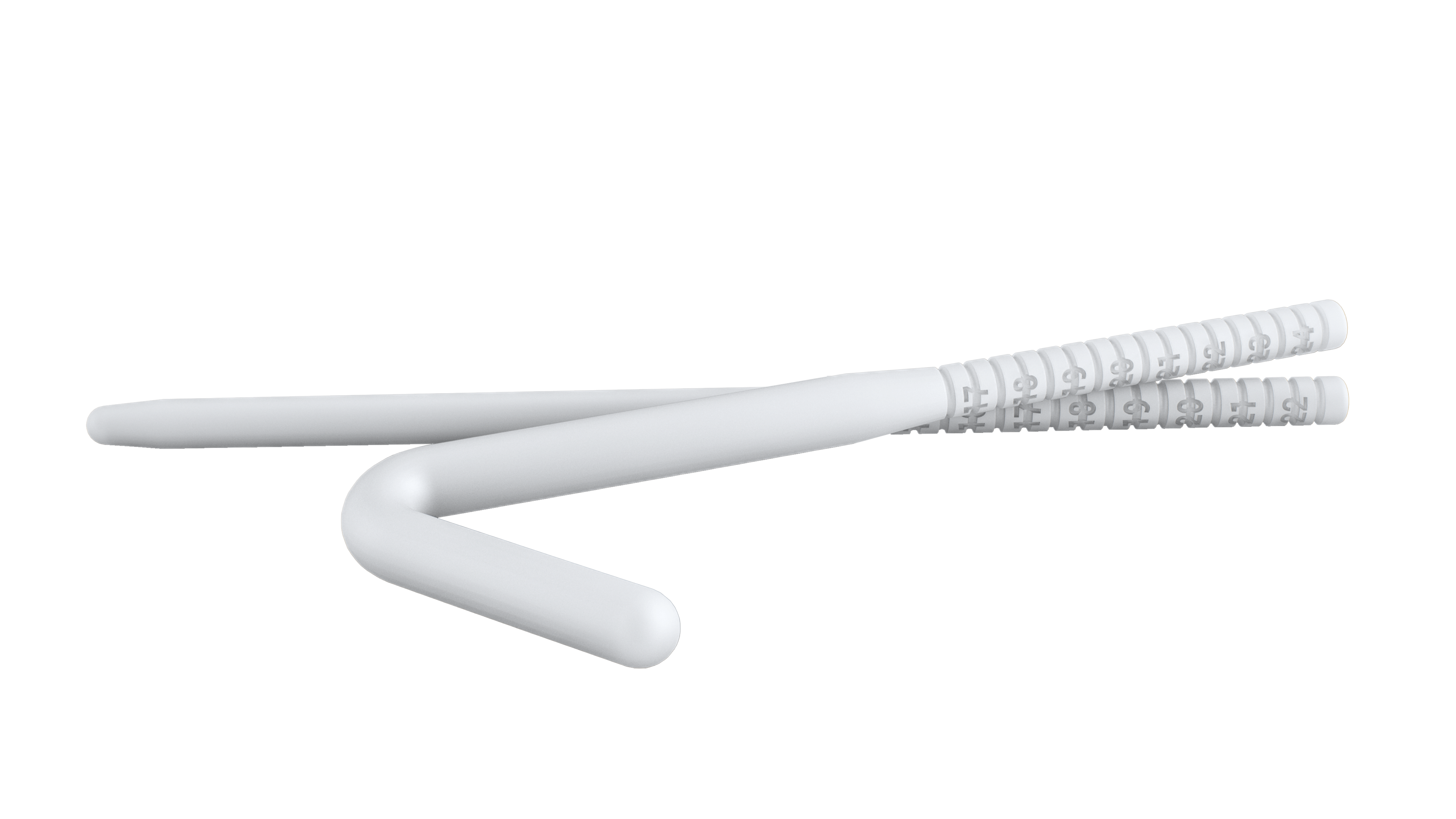
- Malleable semi-rigid rods, inserted through a small penile incision. Flexible wires allow the device to be straight for penetration or curved for concealment.
- Inflatable penile prosthesis, which provides stiffness and increased girth by pumping saline into balloon-like structures implanted in the penis’ erectile chambers. Currently, only three-piece models are available, as they offer the best results for erection and flaccidity.
Can someone who views me without clothing know that I have a penile prosthesis?
No, the device is invisible when integrated into the genitalia.
How long will my erection last with a penile prosthesis?
Your erection is on-demand and can be maintained for as long as you desire. Flaccidity is also under your control.
Will I have an orgasm with a penile prosthesis?
The penile implant does not impact orgasm or ejaculation. These events occur following sexual excitement. If you could achieve orgasm or ejaculation before the penile implant surgery, you should be able to do so afterward.
Can I impregnate a woman with a penile prosthesis?
Yes, if the woman is fertile and you have not undergone sterilization, sex with a penile implant can result in pregnancy. Many young men with diabetes seek penile implant surgery to restore their ability to father a child.
Can I have an imaging procedure called MRI with a penile implant?
Yes, all penile implant devices are MR Conditional, meaning you can be safely scanned. Manufacturers provide special instructions for those with a penile implant requiring an MRI scan.
What are the benefits of a Rigicon Rigi10™ when compared to an inflatable penile implant?
The Rigicon Rigi10™ malleable device is more affordable, simpler, and less likely to require revision surgery due to malfunction or medical issues. The surgery and recovery time are shorter compared to inflatable penile implants. It is the preferred choice for patients with limited manual dexterity.
What is Rigi10™’s Flexible Rod Technology™?
Rigi10™ malleable prostheses feature cutting-edge Flexible Rod Technology™, offering a high bending angle of ≥130º. This high bending angle makes the surgery easier for the surgeon and provides better concealability for the patient.
FAQ’s Disclaimer
The content is not intended to be a substitute for, nor does it replace professional medical advice, diagnosis, or treatment. If you have any concerns or questions about your health, you should always consult with a physician or other health-care professional.
-
*Rigi10™ Malleable Penile Prosthesis 14 mm is not available for sale within the United States.
-
Federal law (USA) restricts this device to sale by or on the order of a physician.