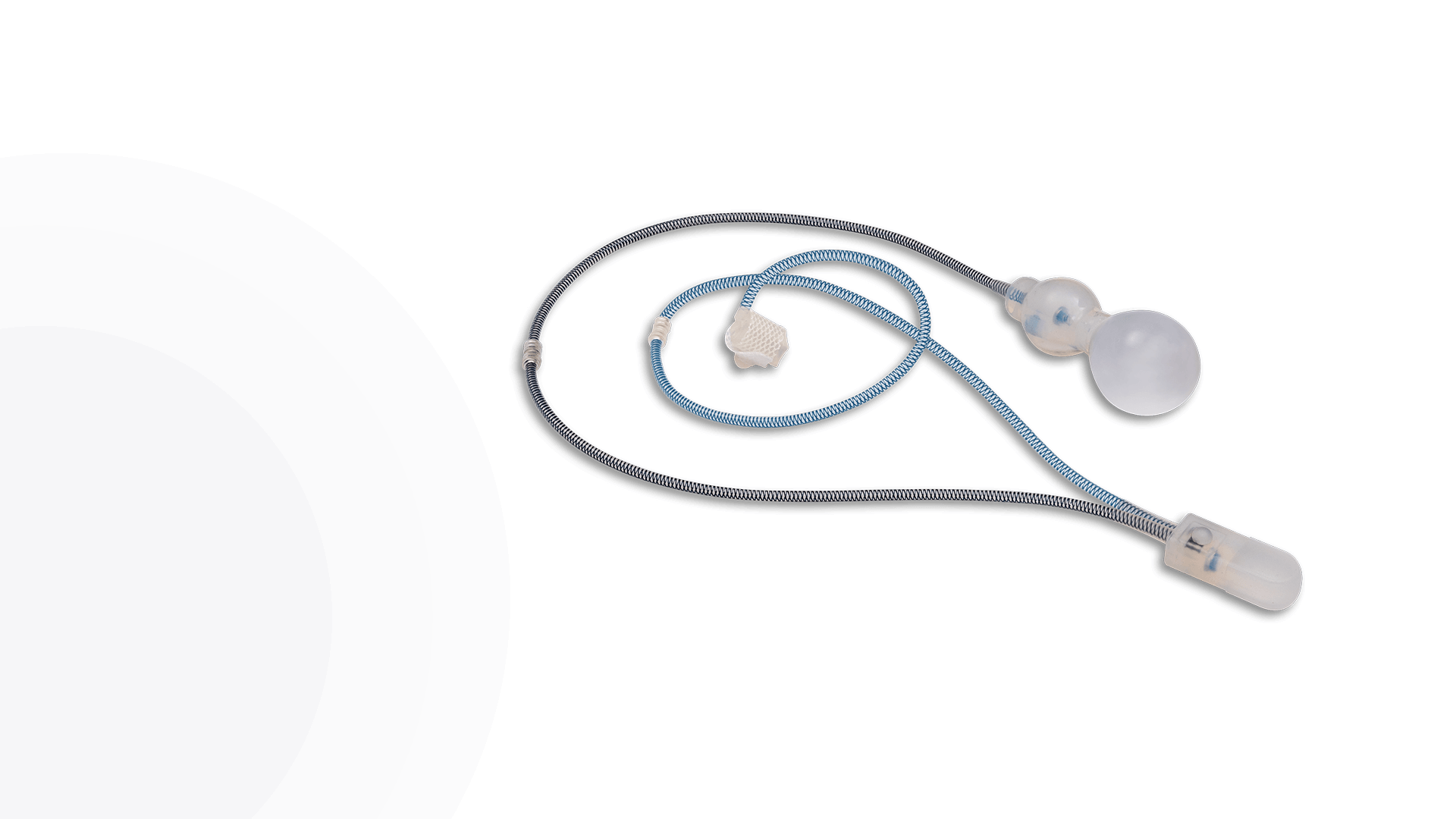
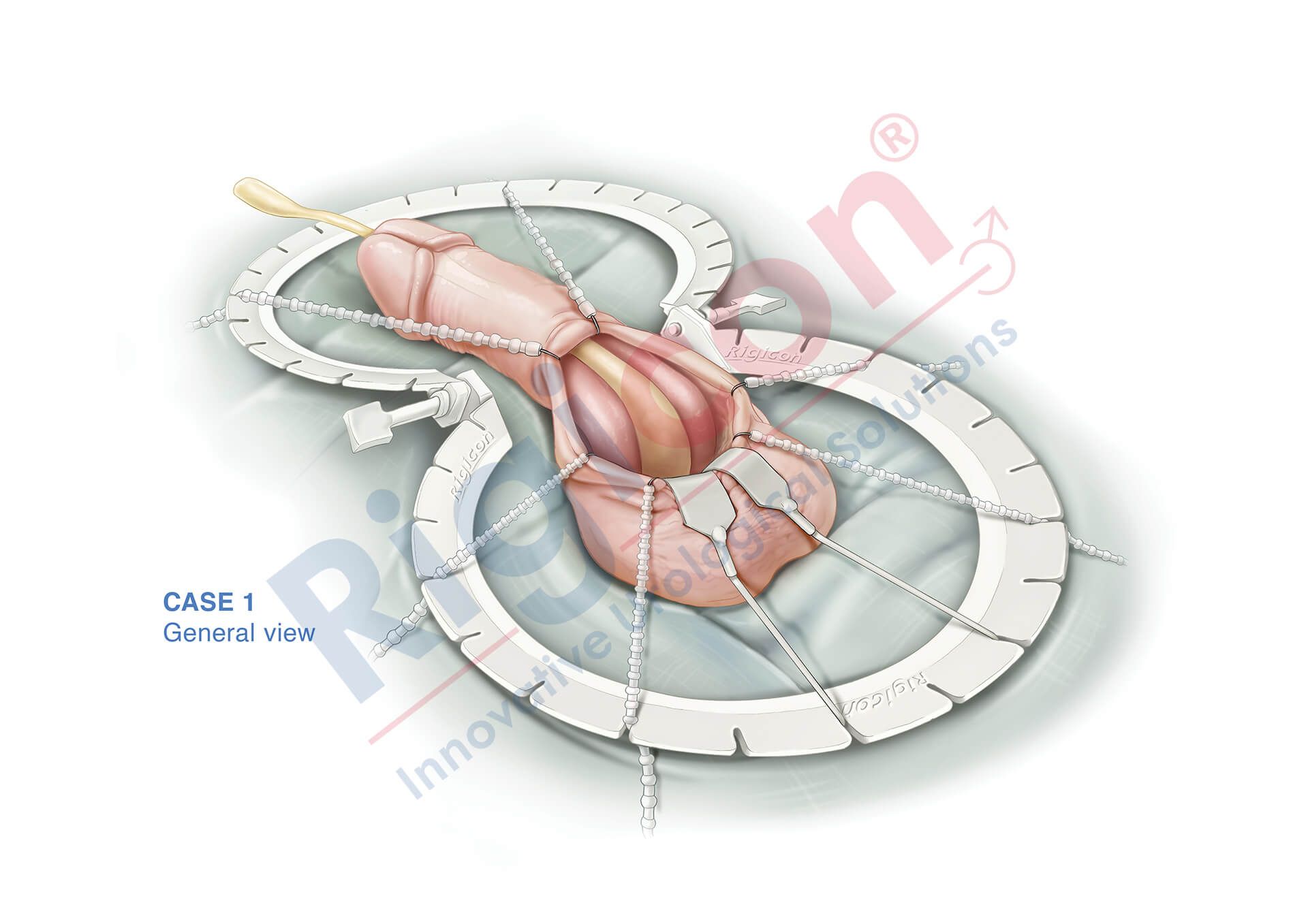
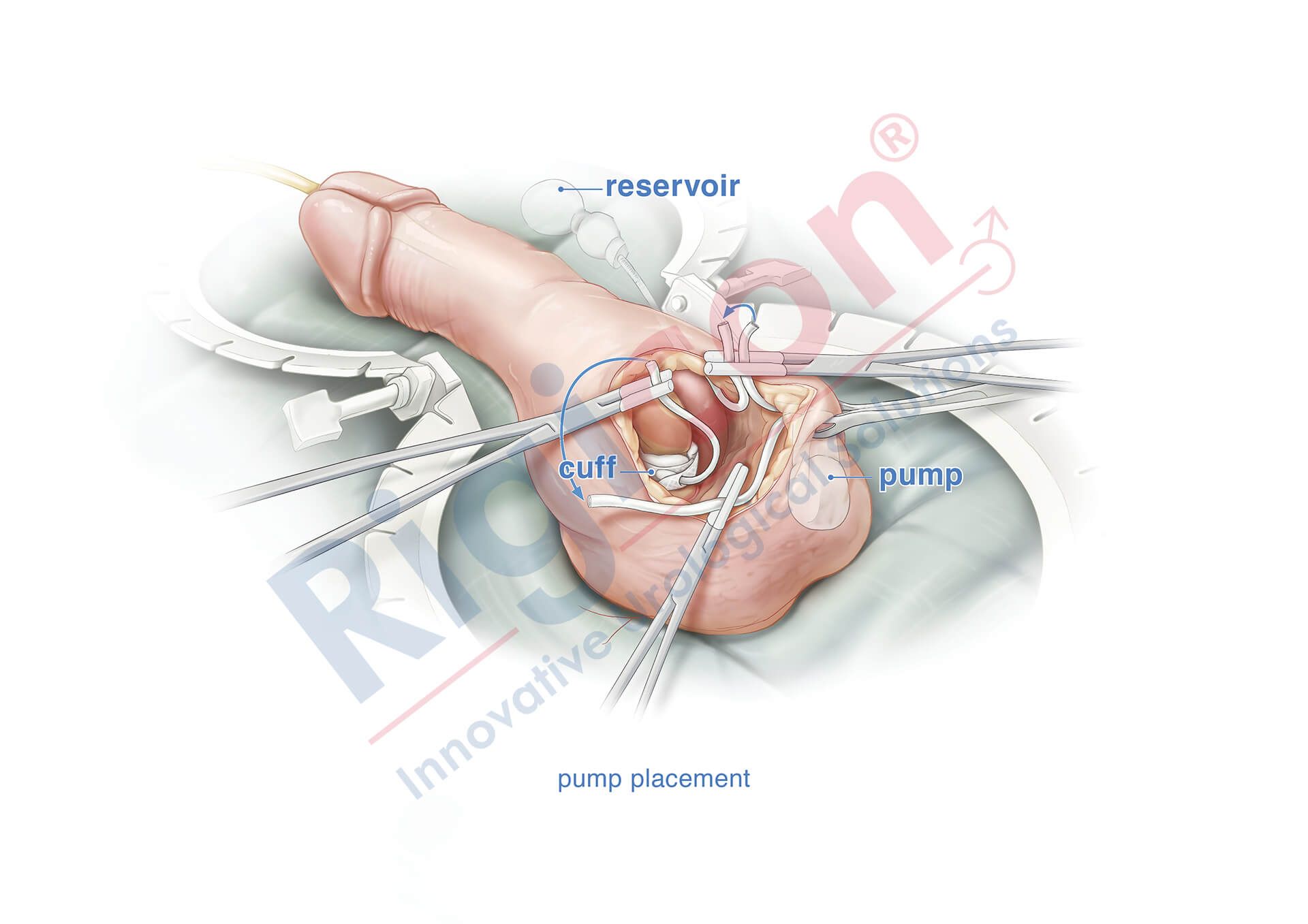
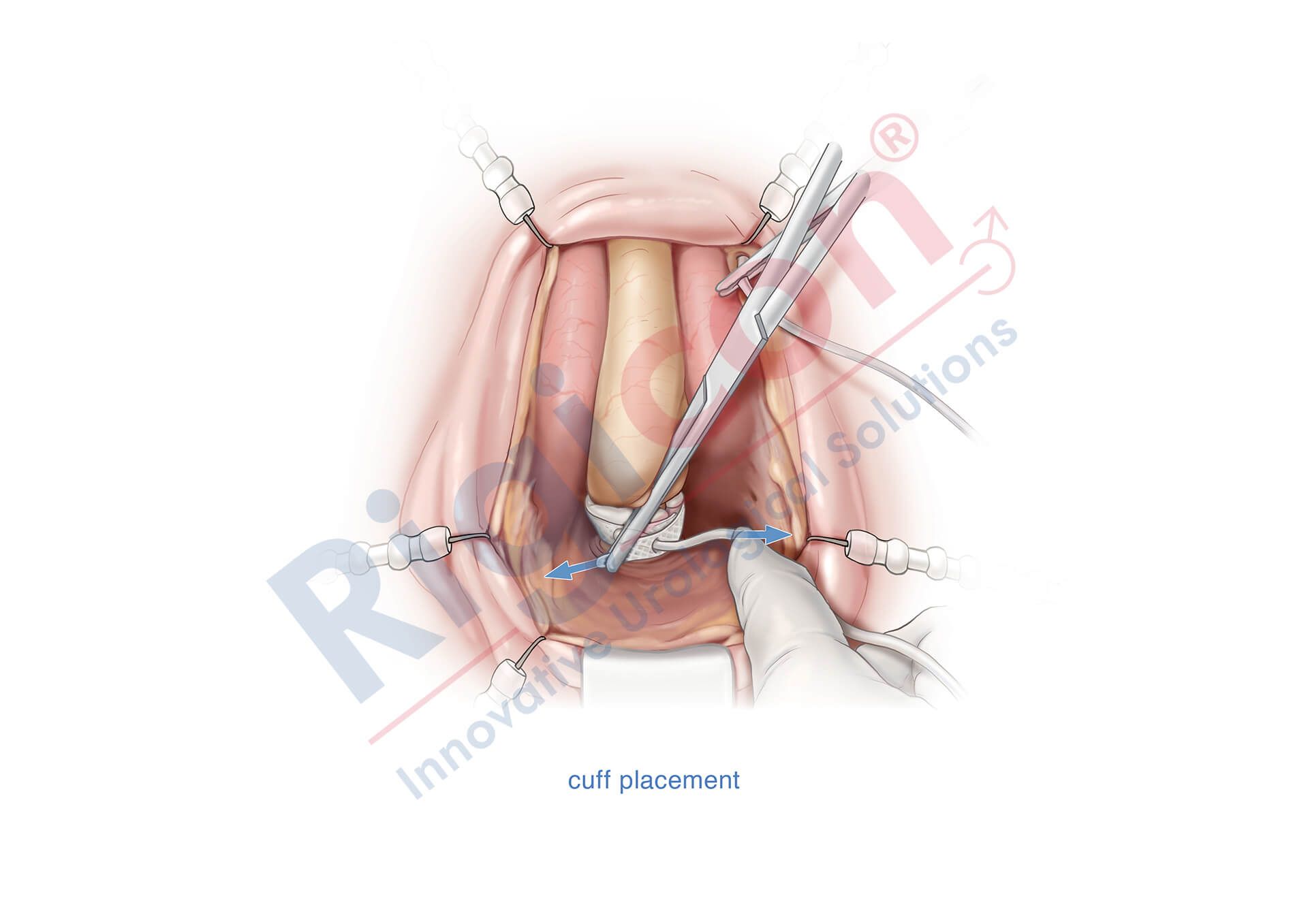
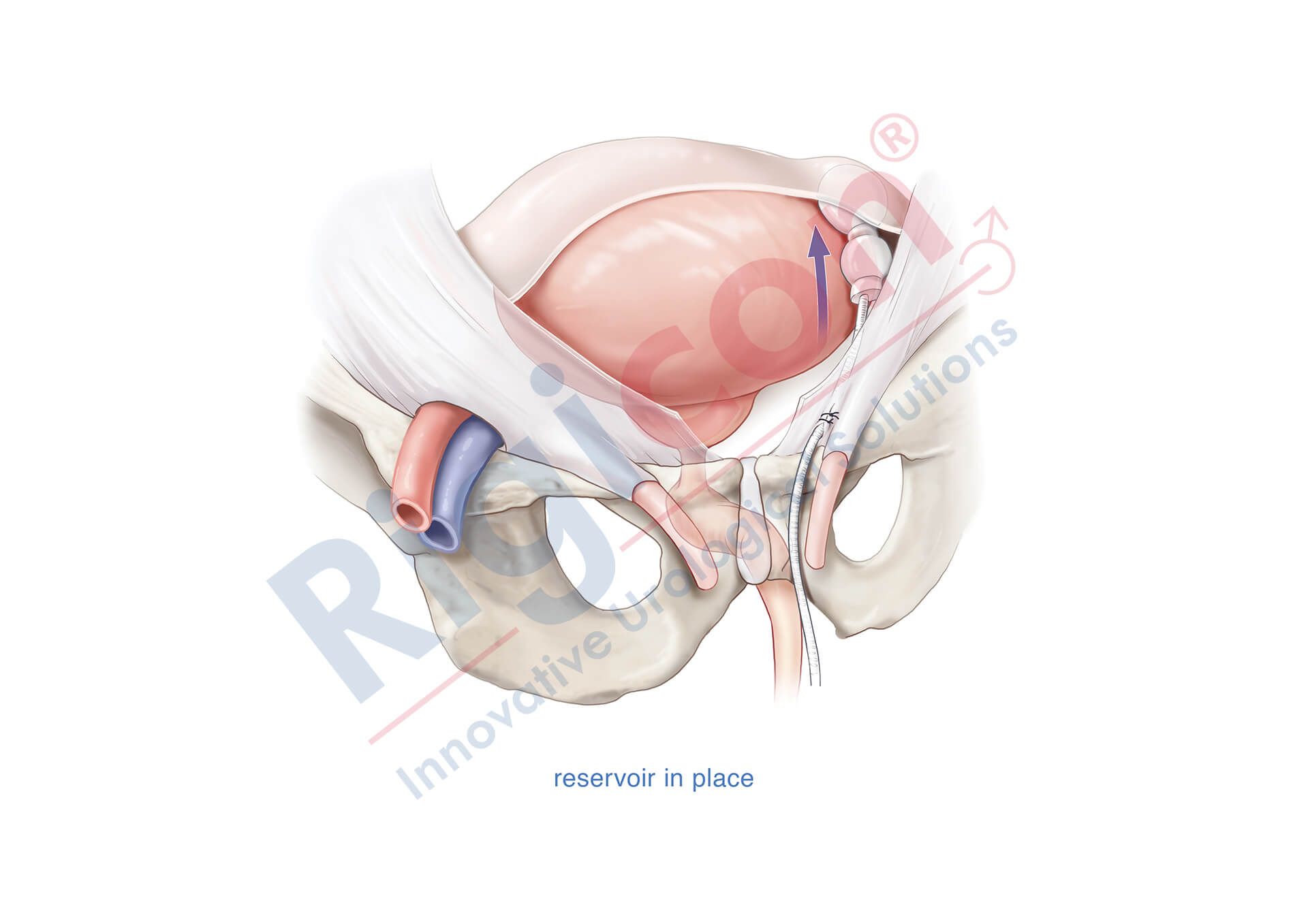
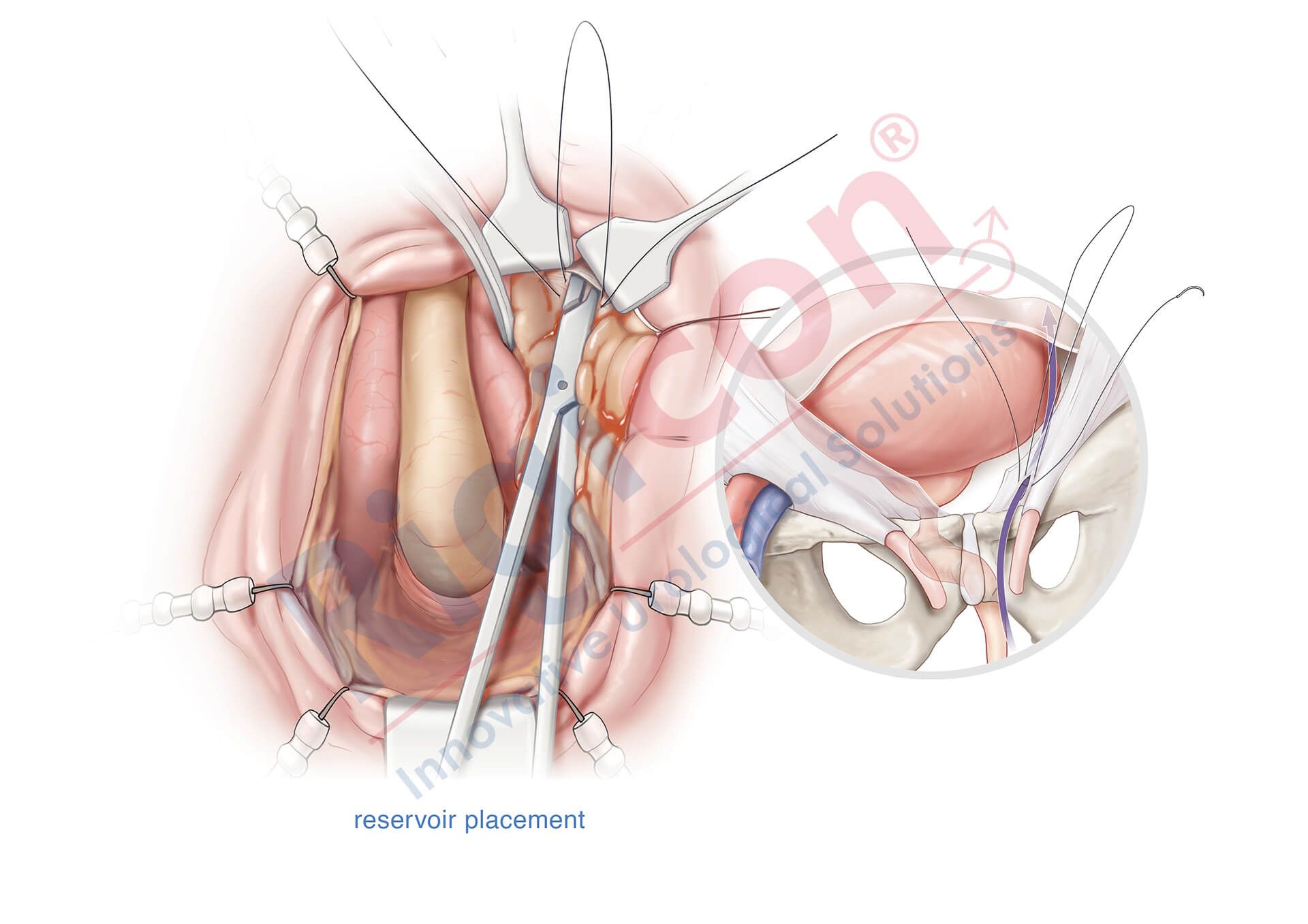
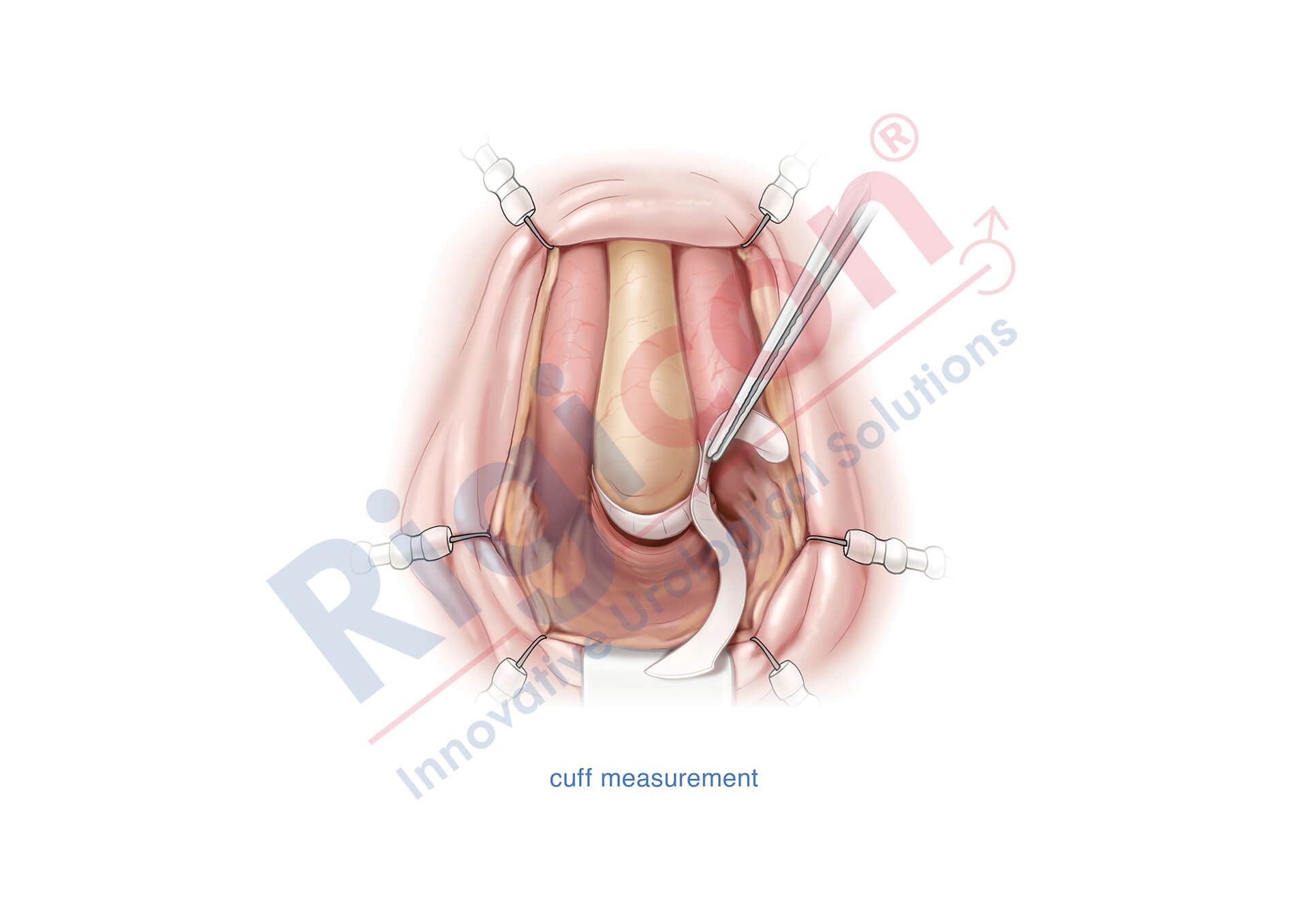
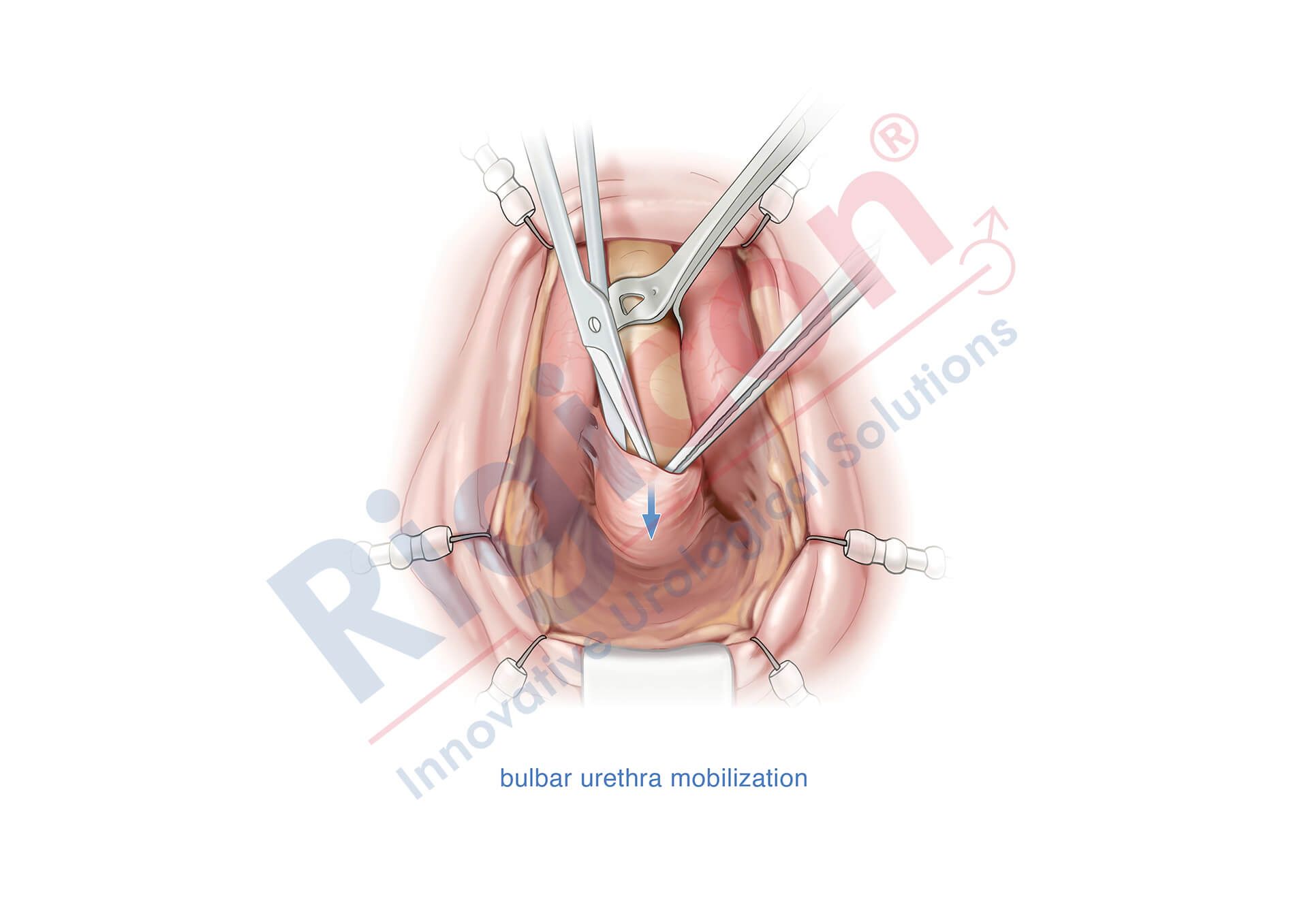
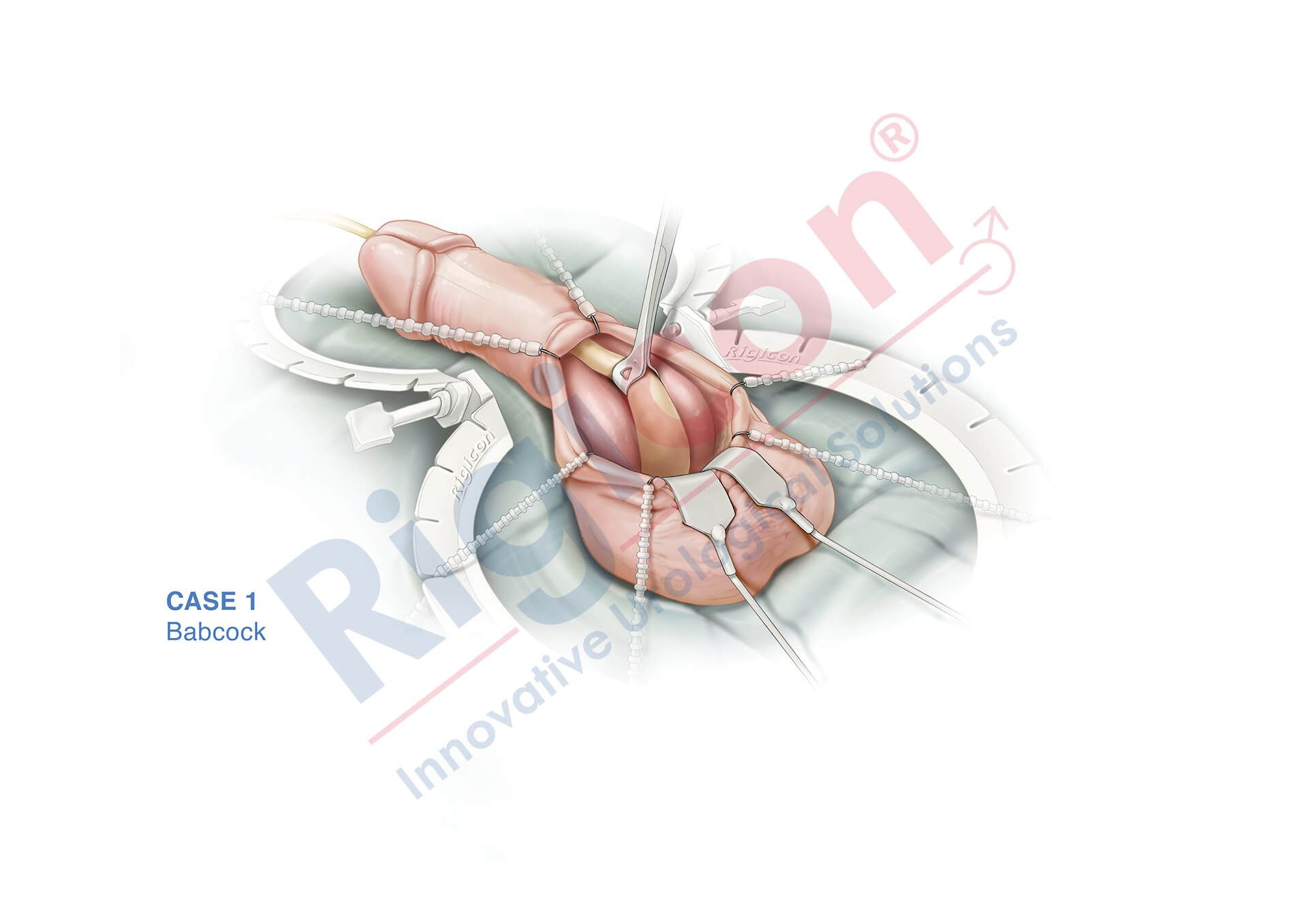
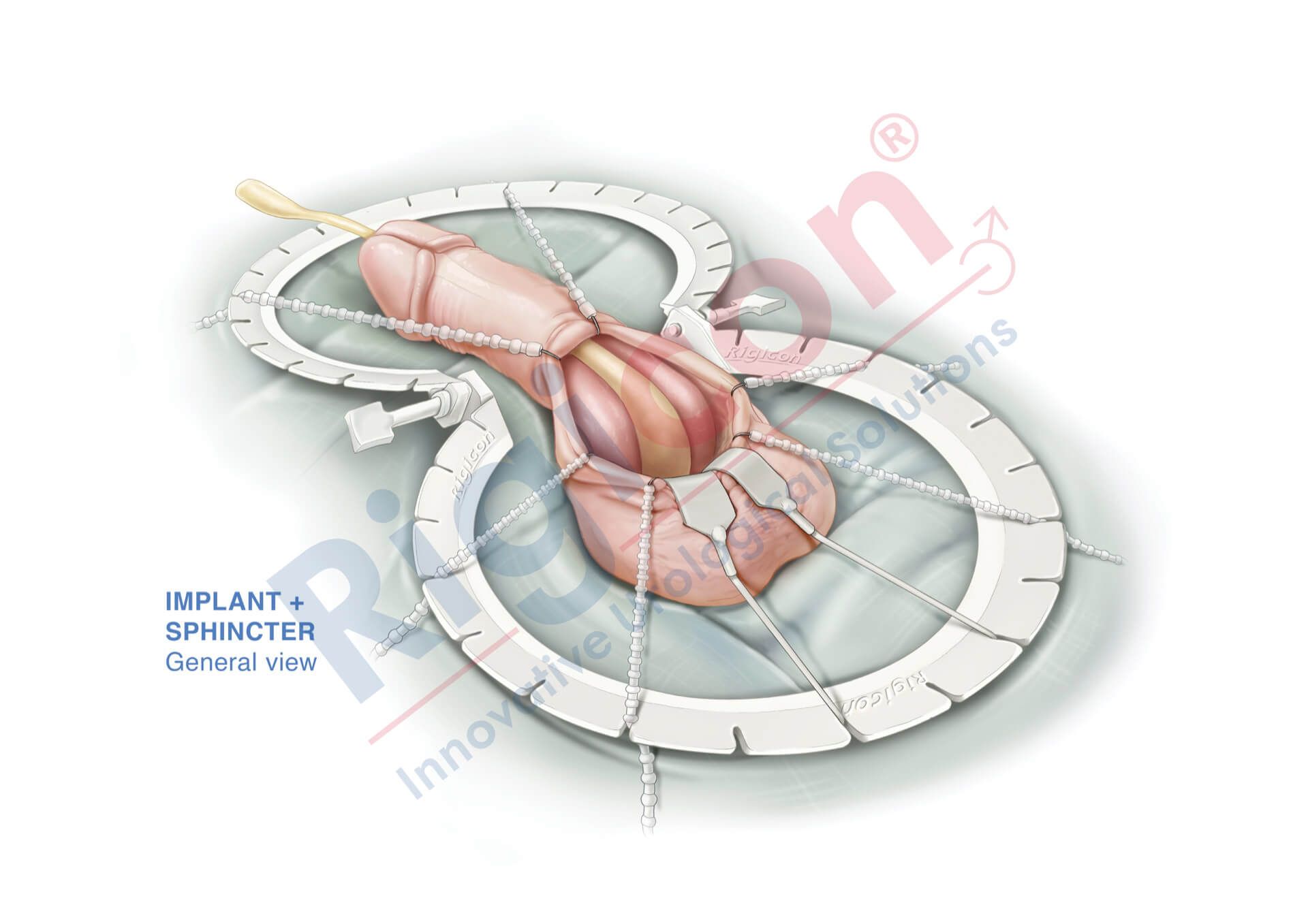
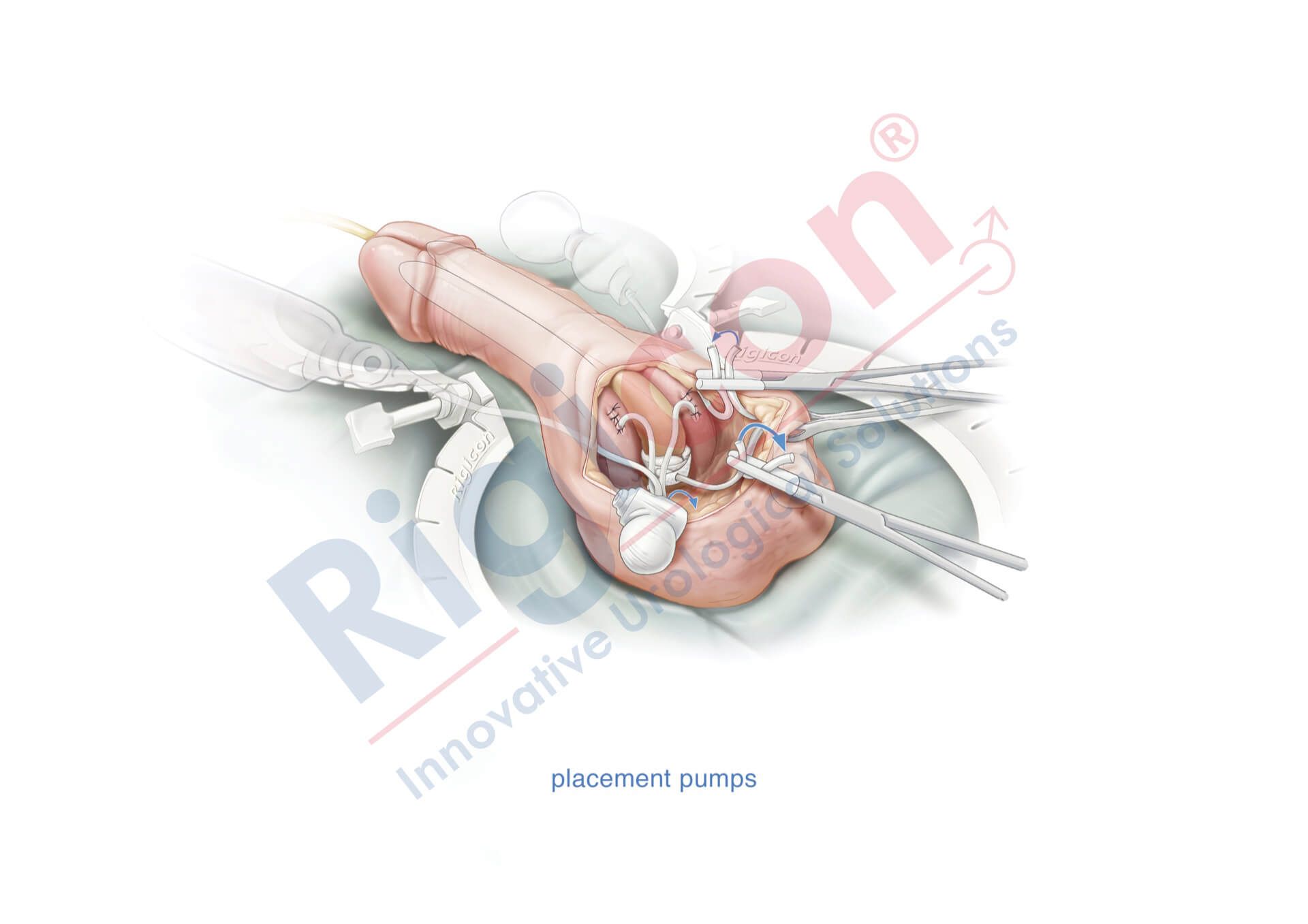
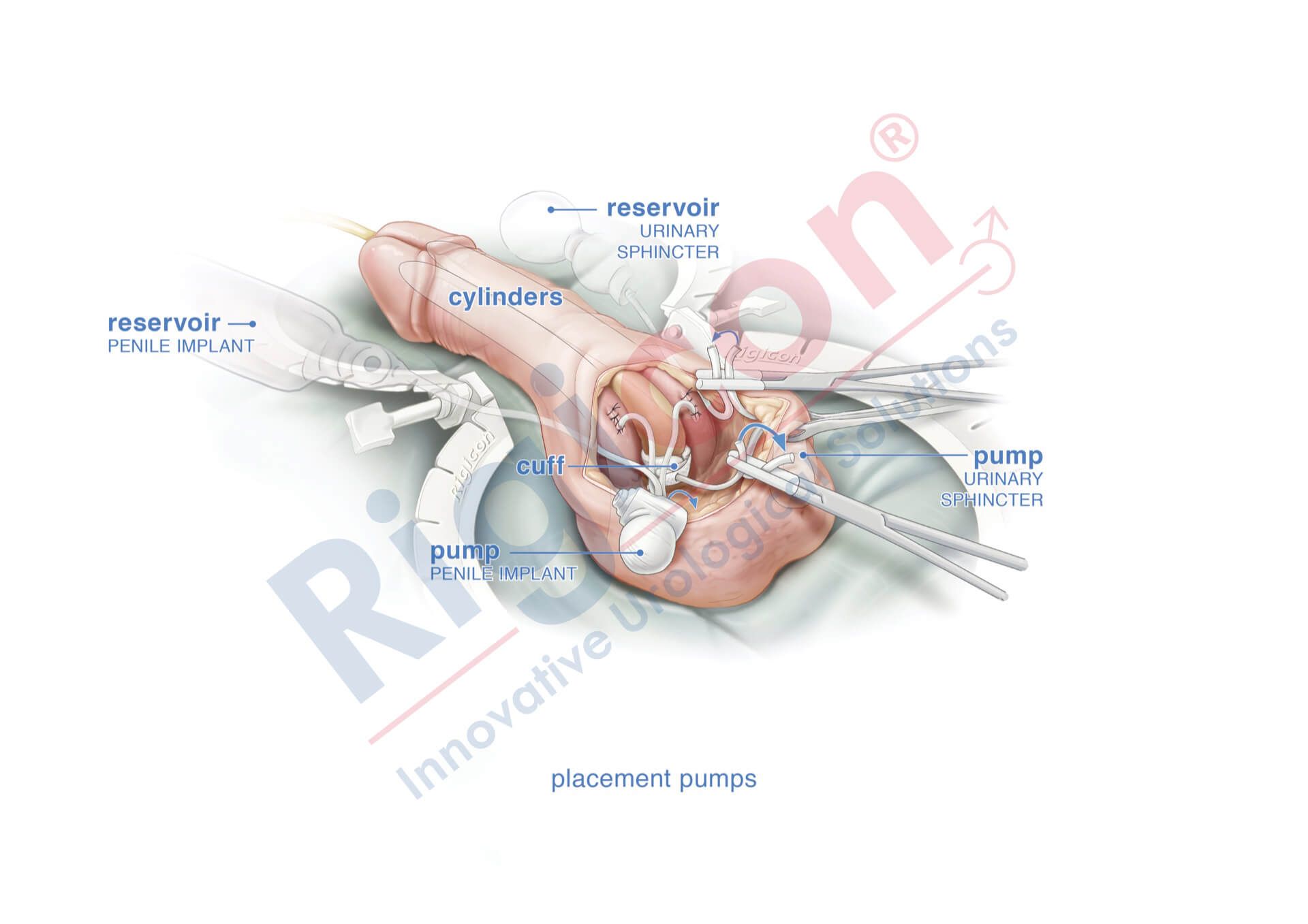
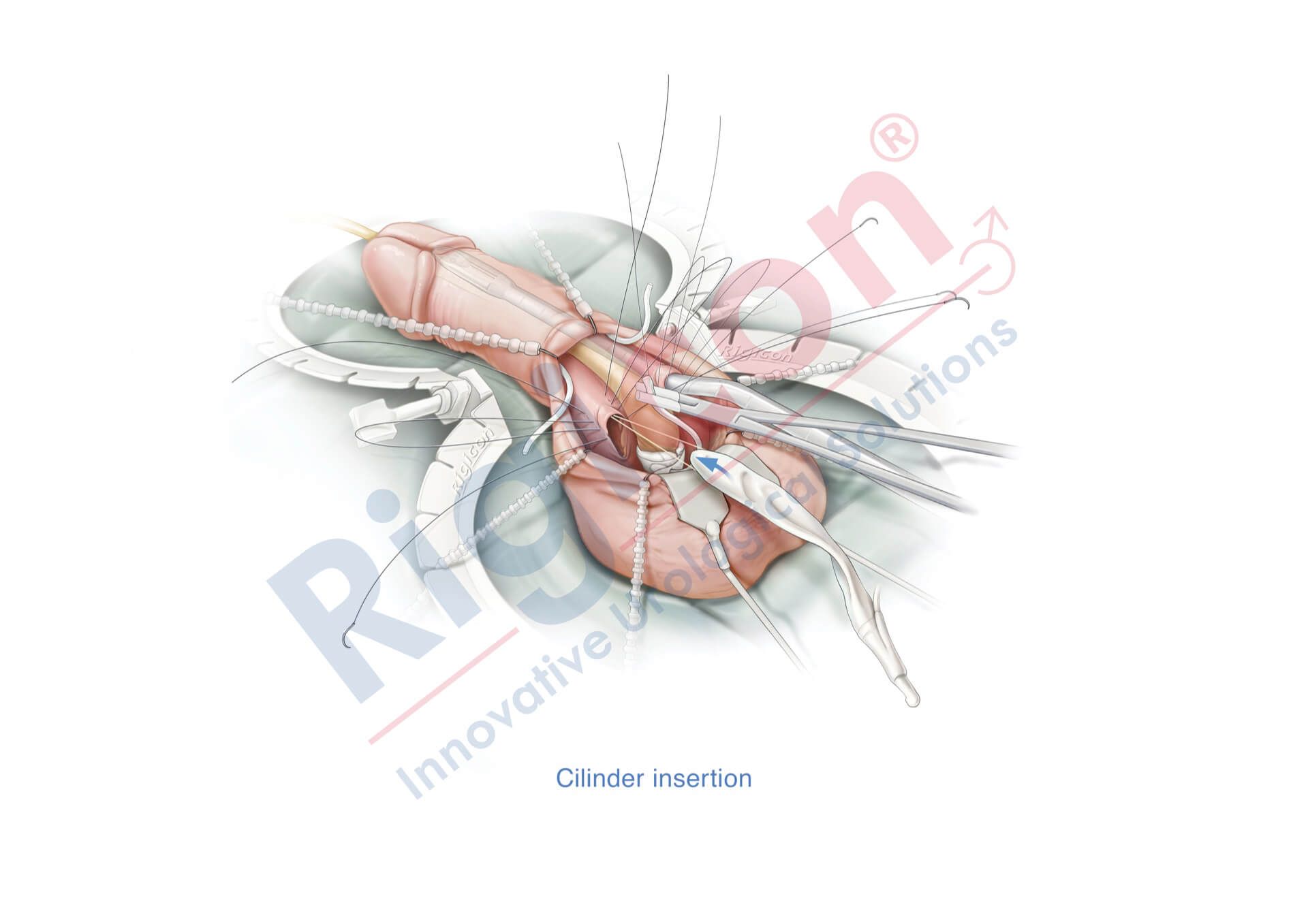
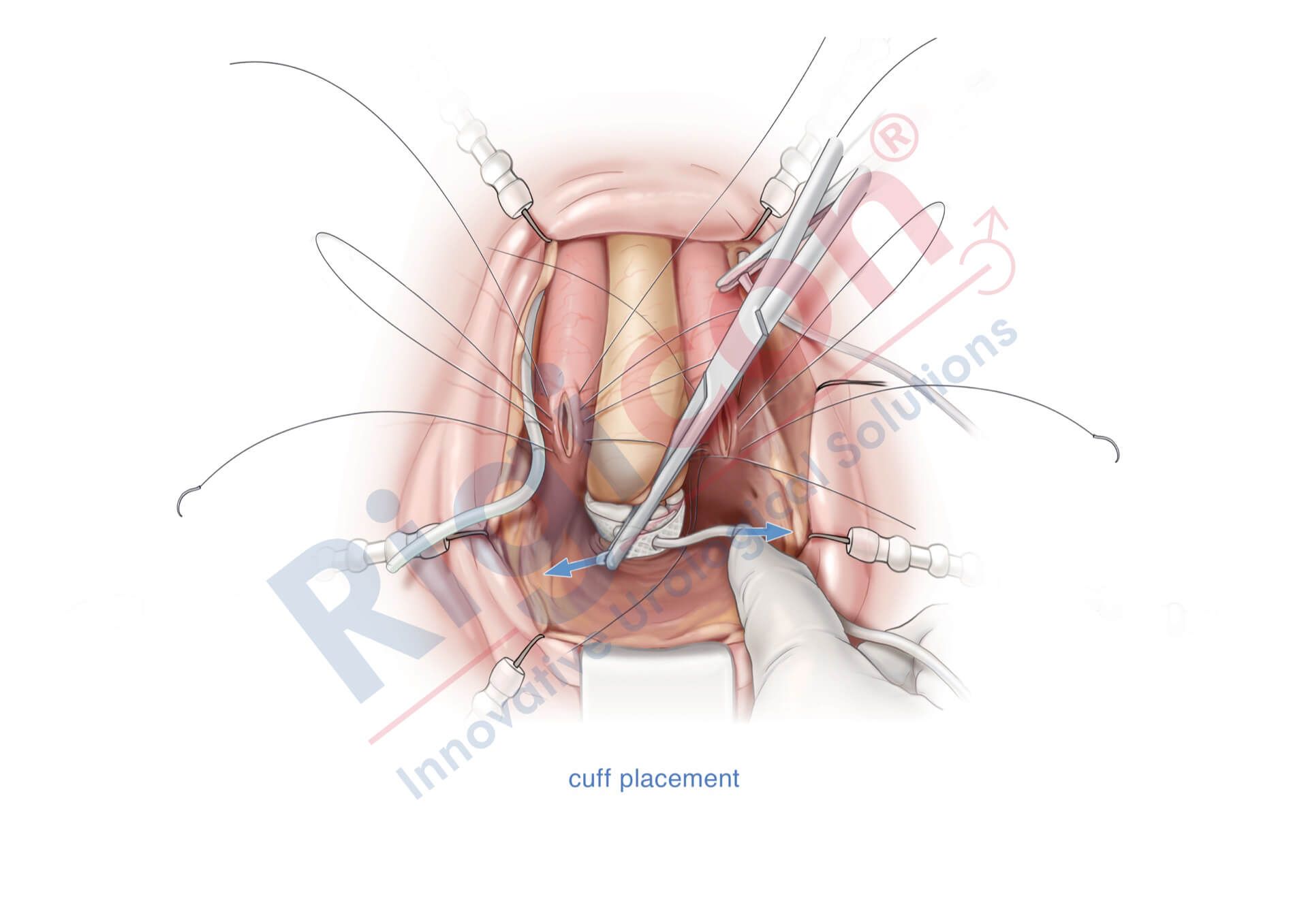
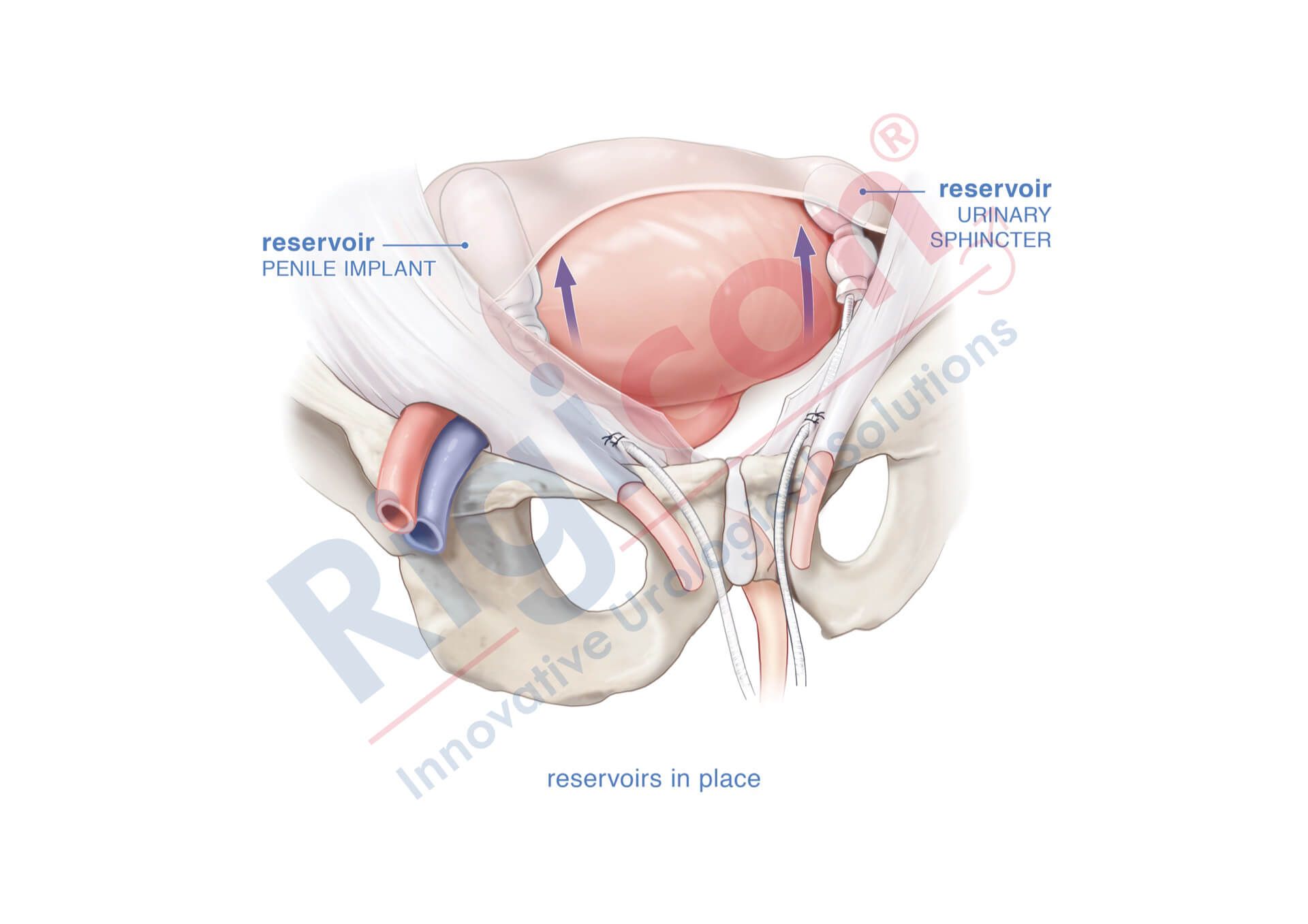
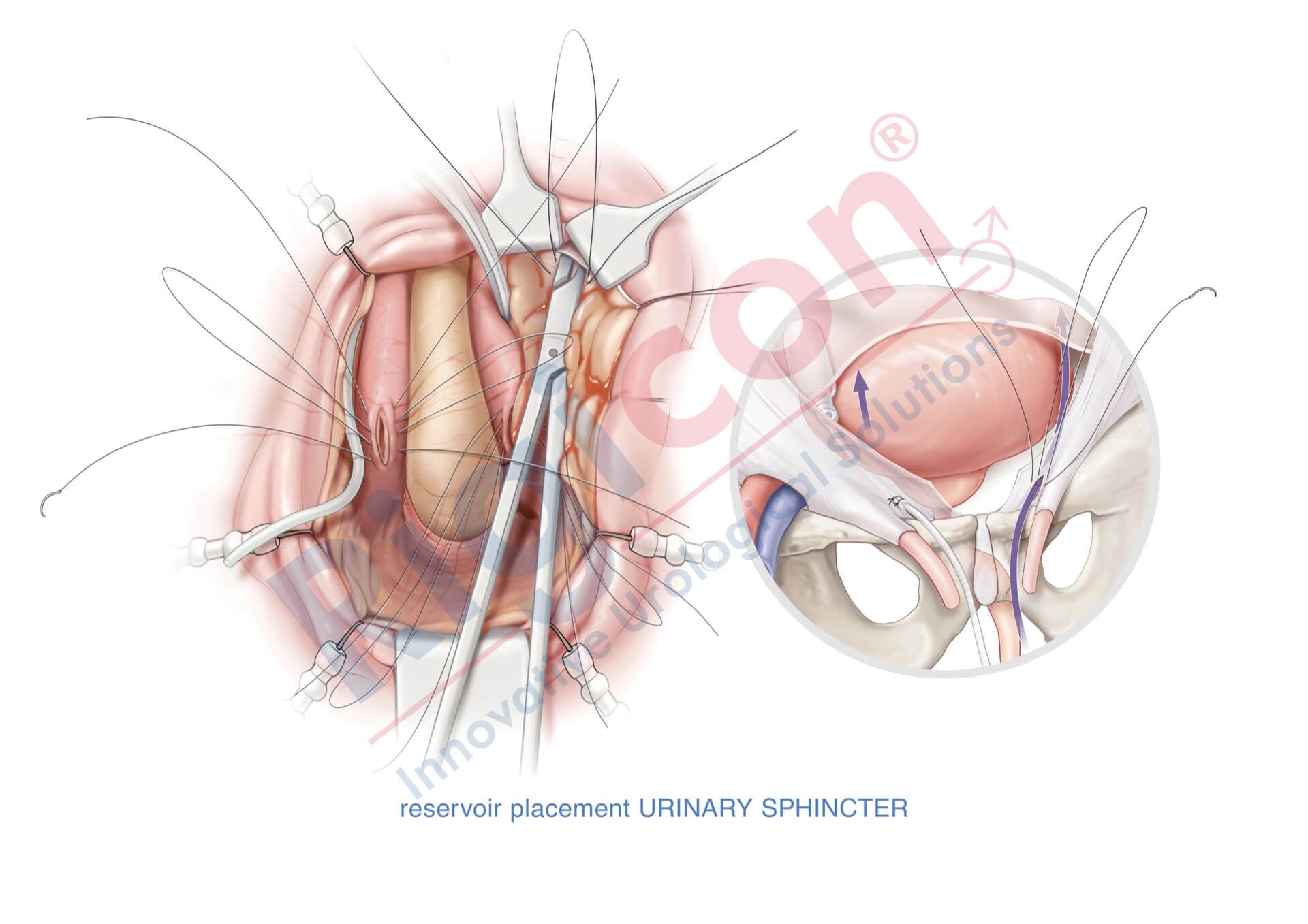
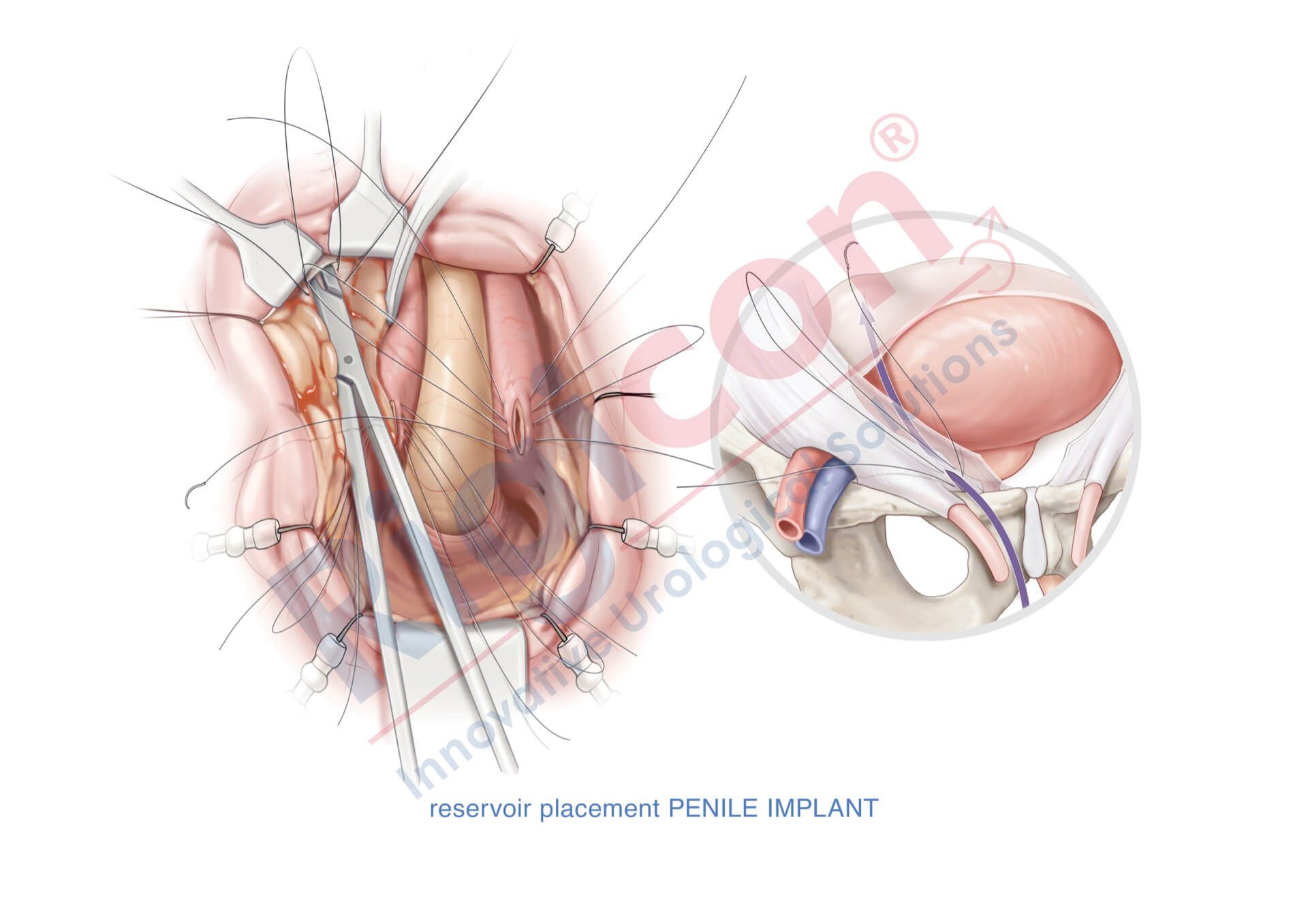
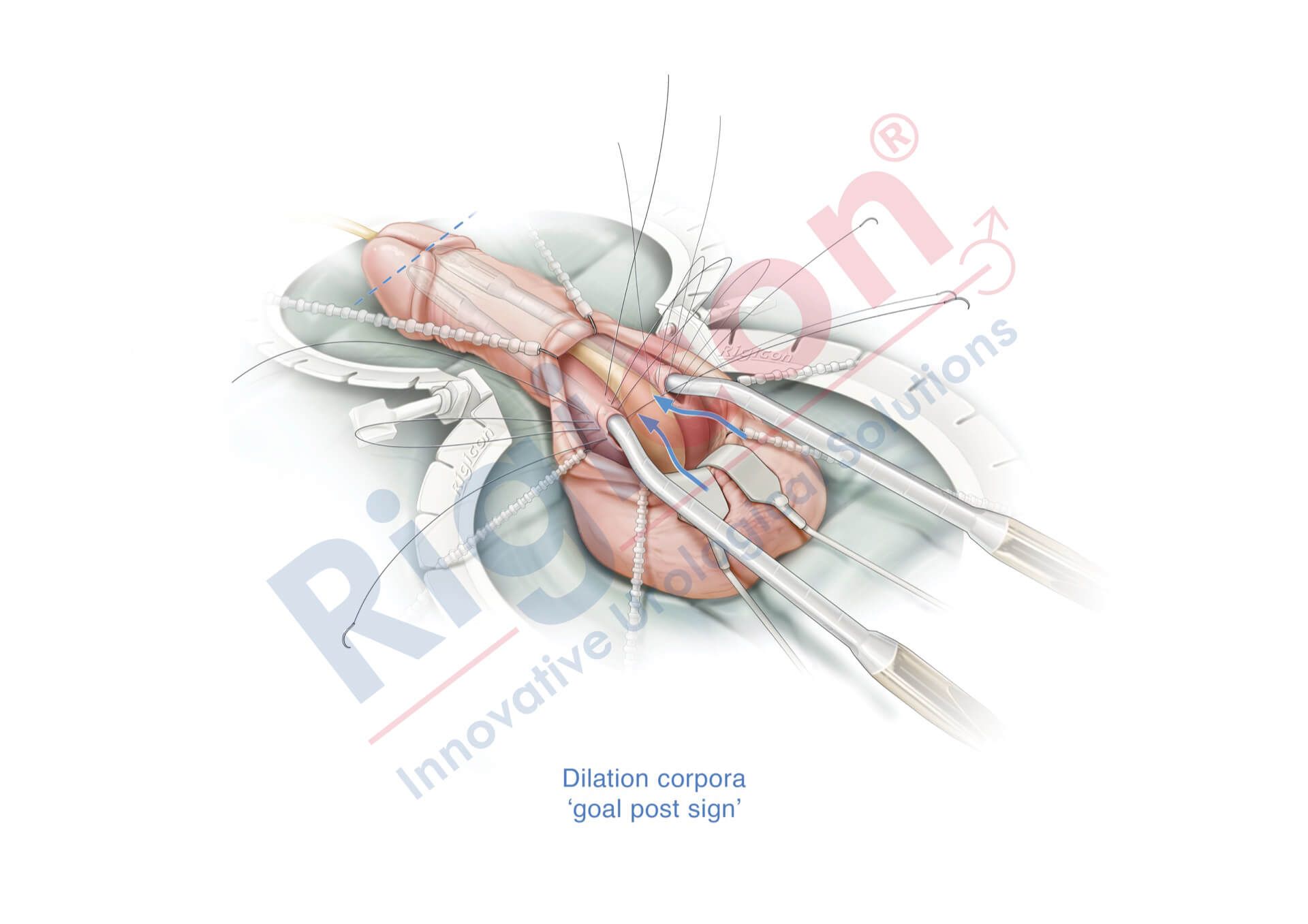
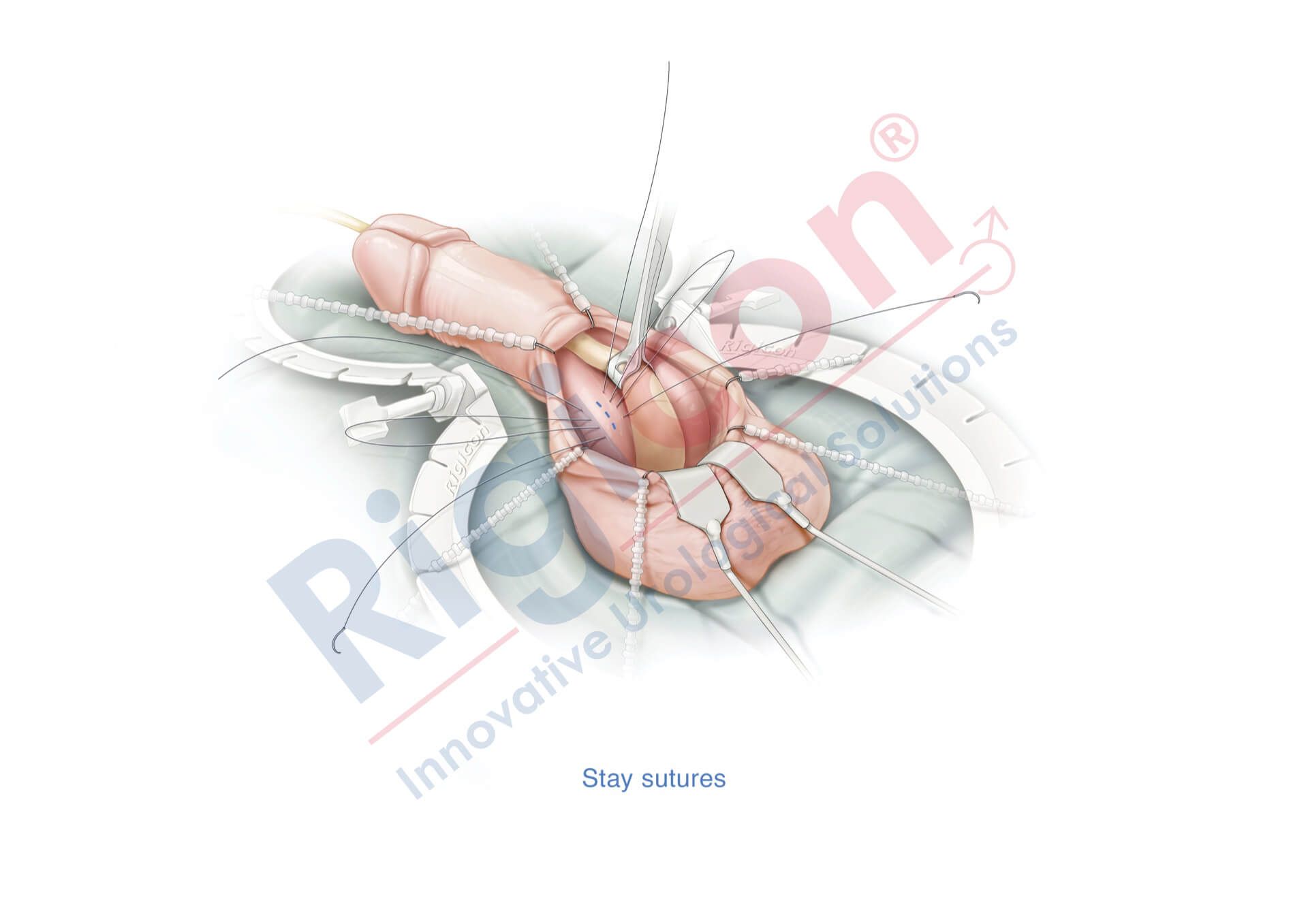
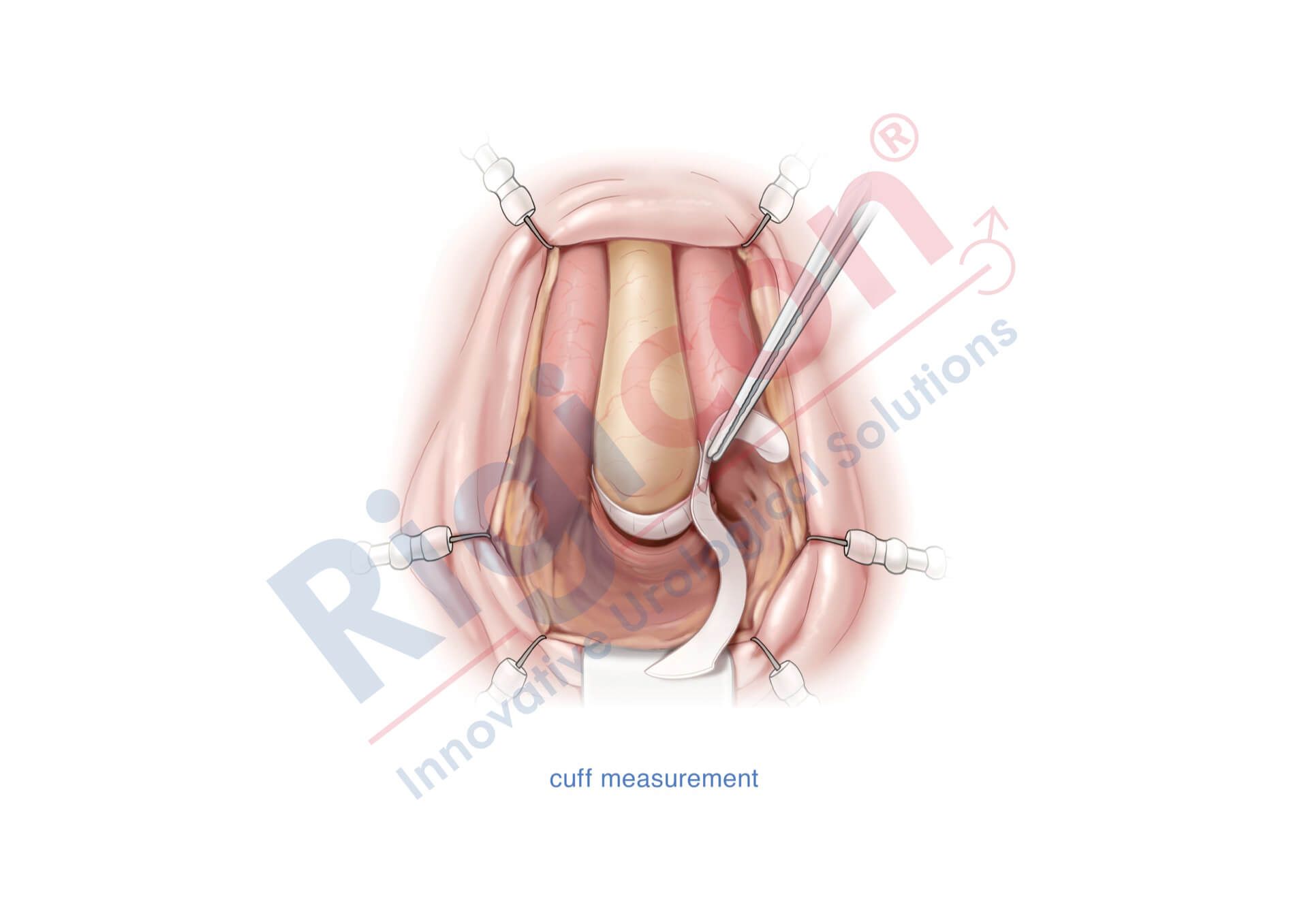
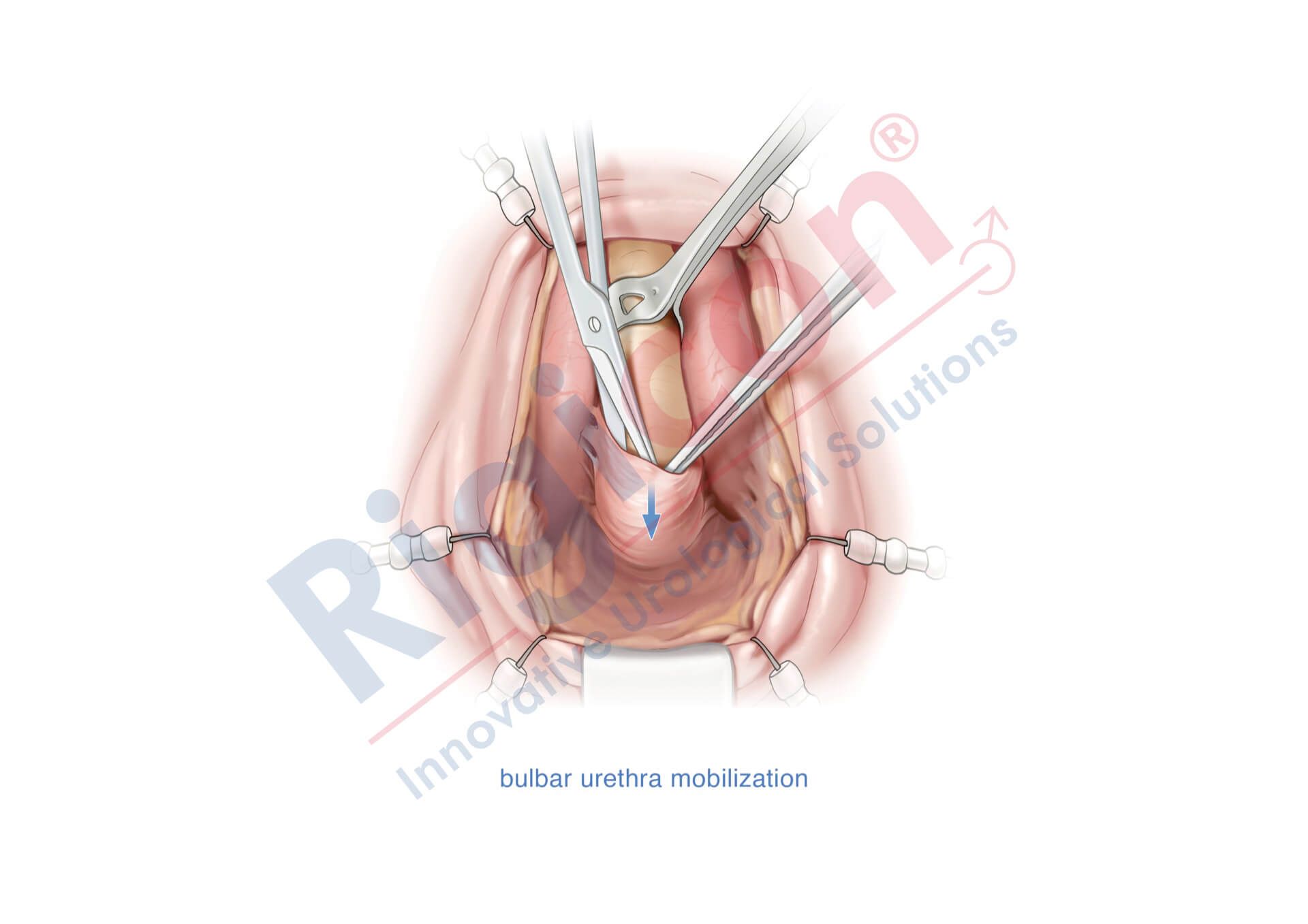
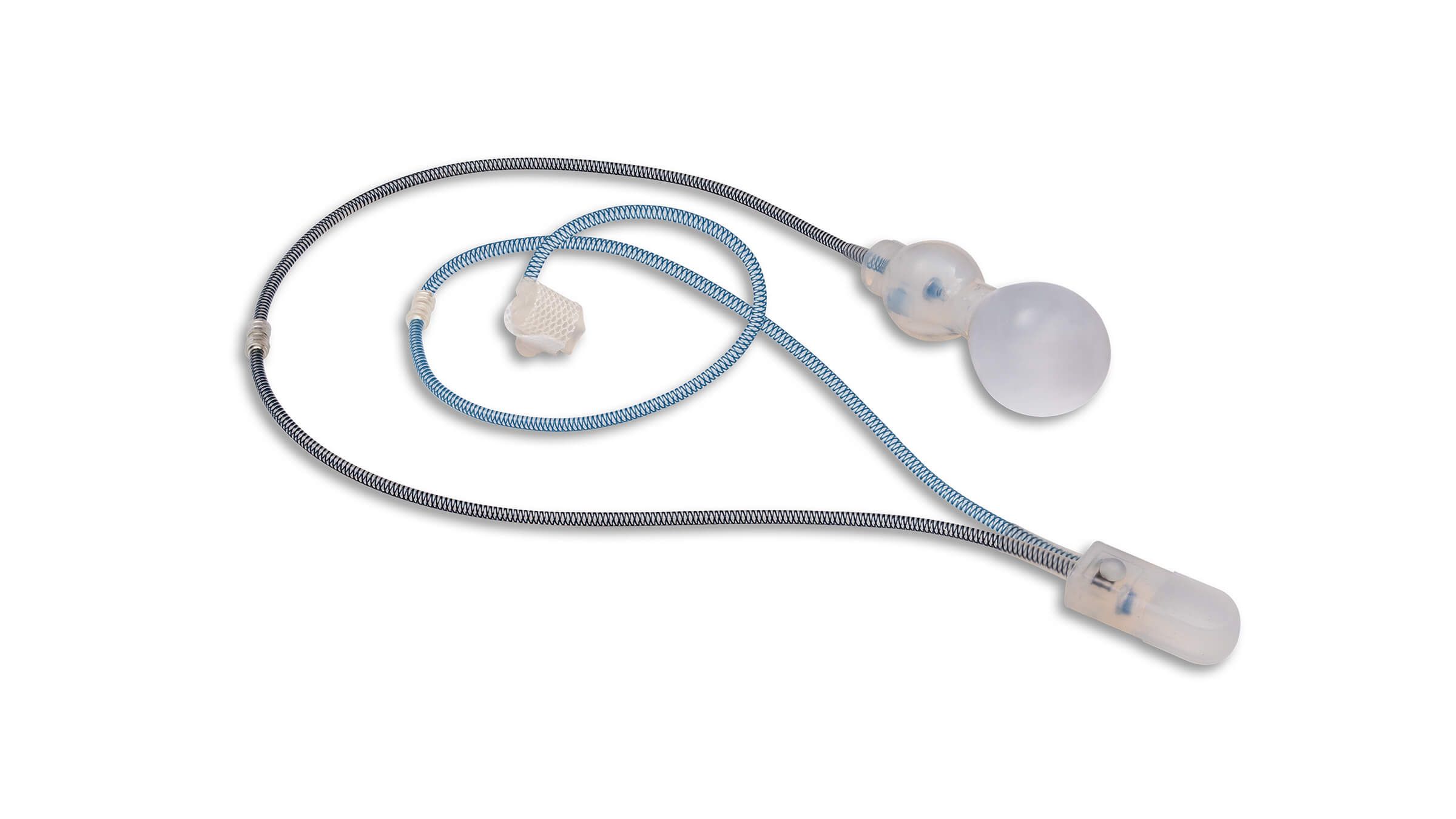
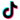ContiReflex® Enhanced Artificial Urinary Sphincter is used to treat urinary incontinence due to intrinsic sphincter deficiency in cases such as incontinence following prostate surgery.4
Unlike traditional devices, the ContiReflex® with its smart reflex balloon constantly senses intra-abdominal pressure changes and simultaneously adapts the pressure on the urethra, potentially helping patients stay dry more consistently.4
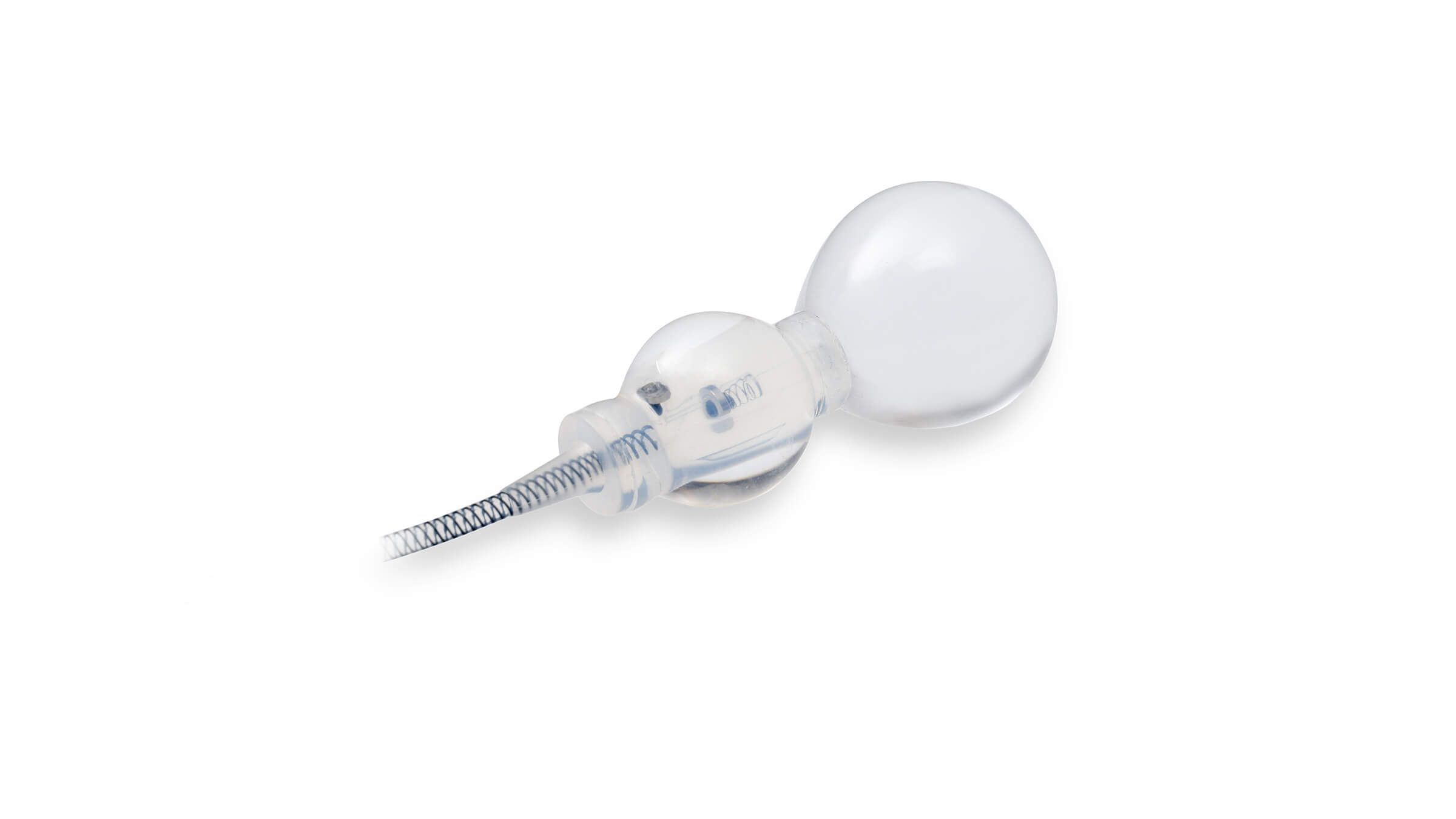
The Reflex Balloon, with its dual component design, allows adjustment of pressure on the urethra in response to the patient’s sudden intra-abdominal pressure changes, promising improved outcomes with the treatment of stress urinary incontinence. In effect, this means that cuff occlusion pressure is no longer limited by the PRB’s labeled pressure.
The ContiReflex’s unique two-component design allows the system to adjust cuff pressure in real time, based on intra-abdominal pressure changes. This means that it can potentially achieve satisfactory continence outcomes with a lower baseline pressure than traditional devices. The lower baseline pressure also helps to reduce stress on the urethra, which could lead to longer device lifespan and reduced risk of urethral complications.
-
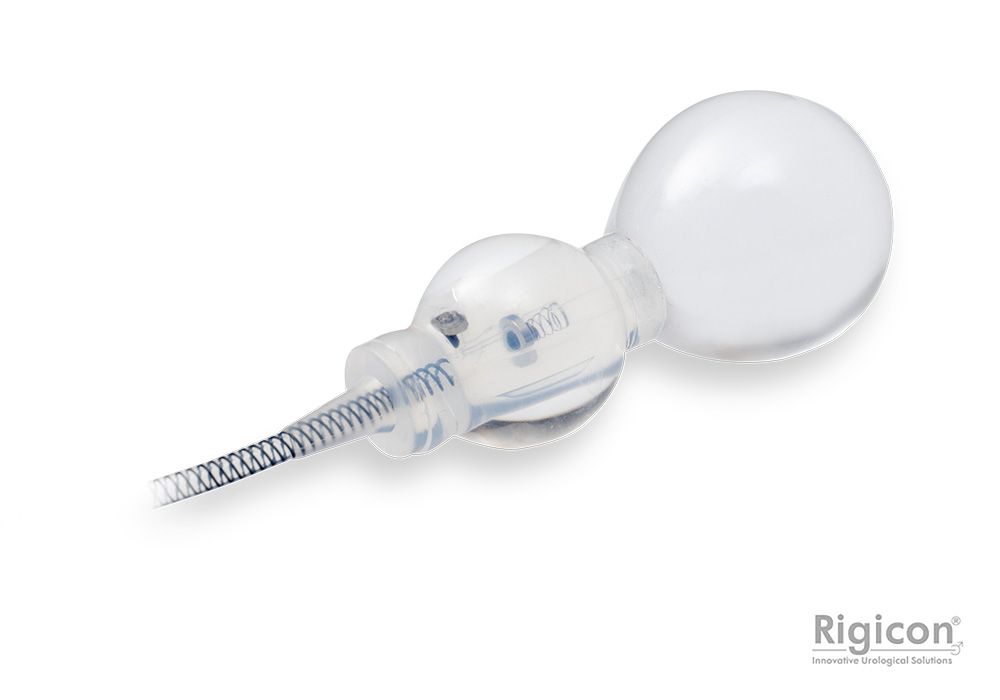
Smart Reflex Balloon
Smart Reflex Balloon senses IAP; changes and modifies occlusive cuff pressure in real-time. Cuff pressure is no longer limited by PRB’s labeled pressure capacity.
- ContiReflex ® PRB Sizes
40 – 49 cm H20 50 – 59 cm H20 60 – 69 cm H20 70 – 79 cm H20 80 – 89 cm H20
- ContiReflex ® PRB Sizes
-
New Cuff Sizes
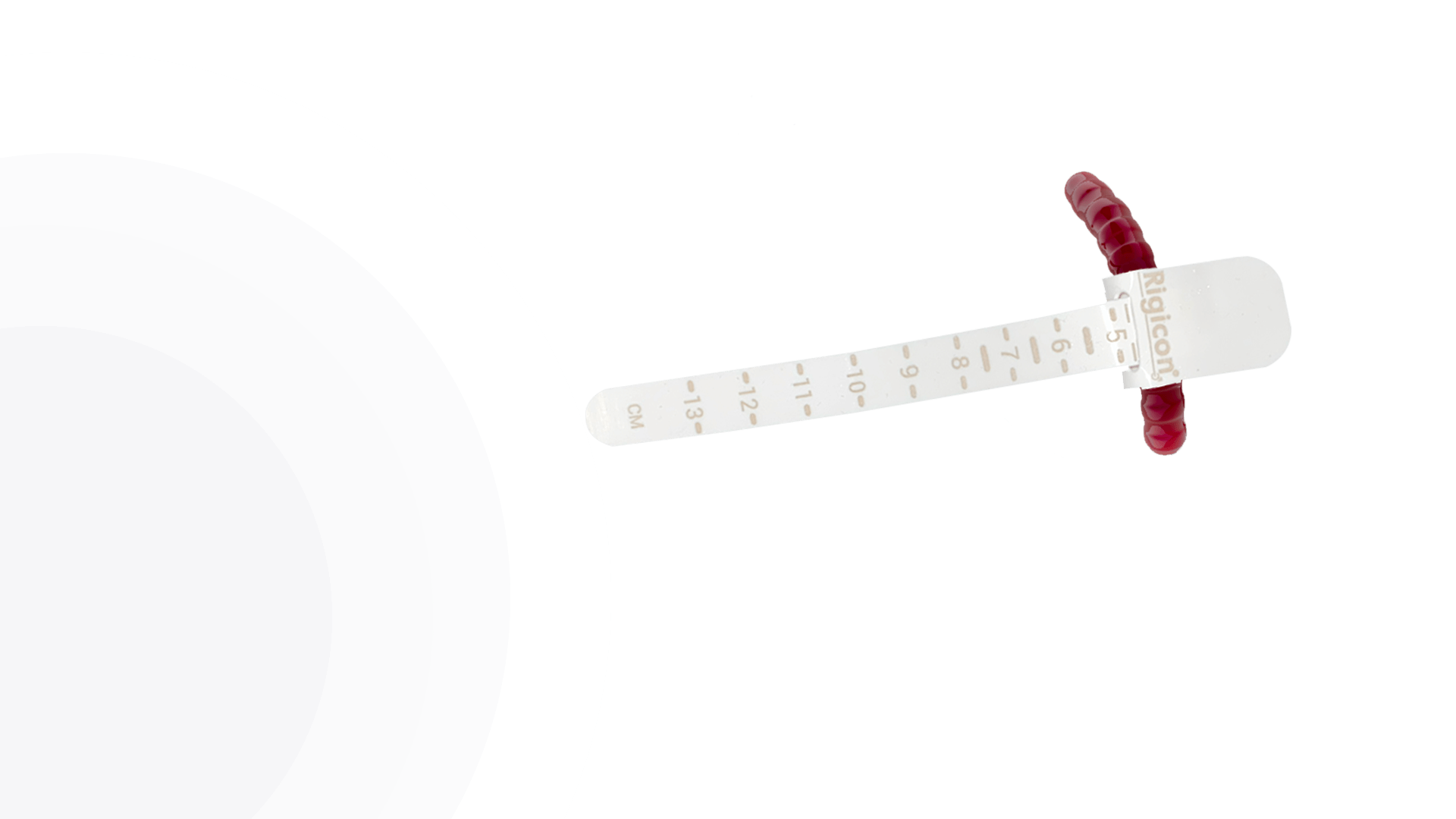
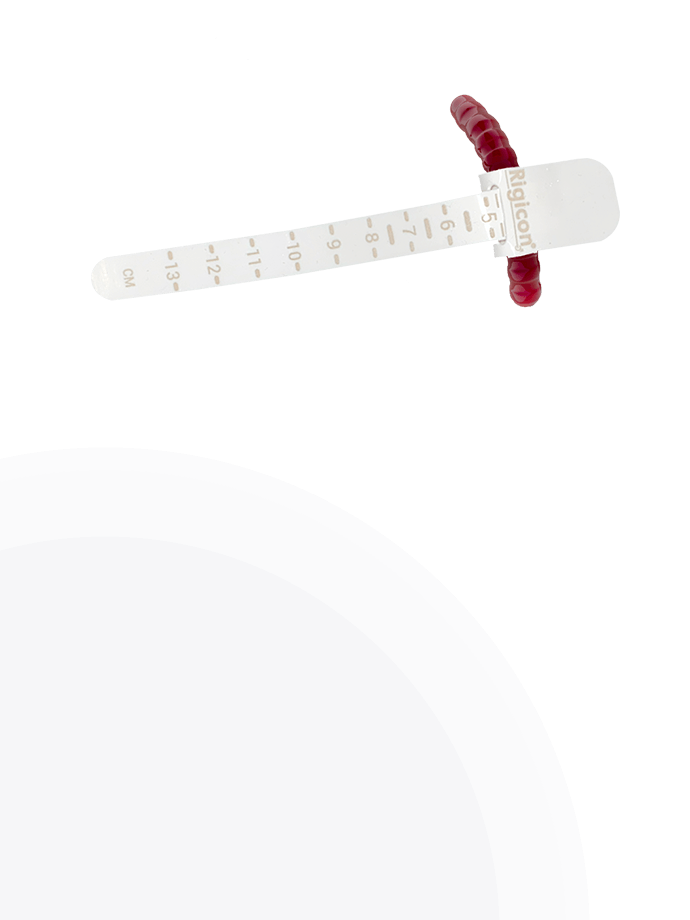
ContiReflex® Enhanced Artificial Urinary Sphincter offers a comprehensive range of occlusive cuff sizes, providing surgeons with unprecedented flexibility in tailoring the device to each patient’s unique urethral anatomy. The diverse selection of cuff sizes spans from 3.5 cm to 13.0 cm, with increments as precise as 0.25 cm in certain ranges, allowing for exceptionally accurate fitting. This extensive array of options enables surgeons to achieve a more personalized and potentially more effective treatment for patients with varying degrees of urinary incontinence.
-
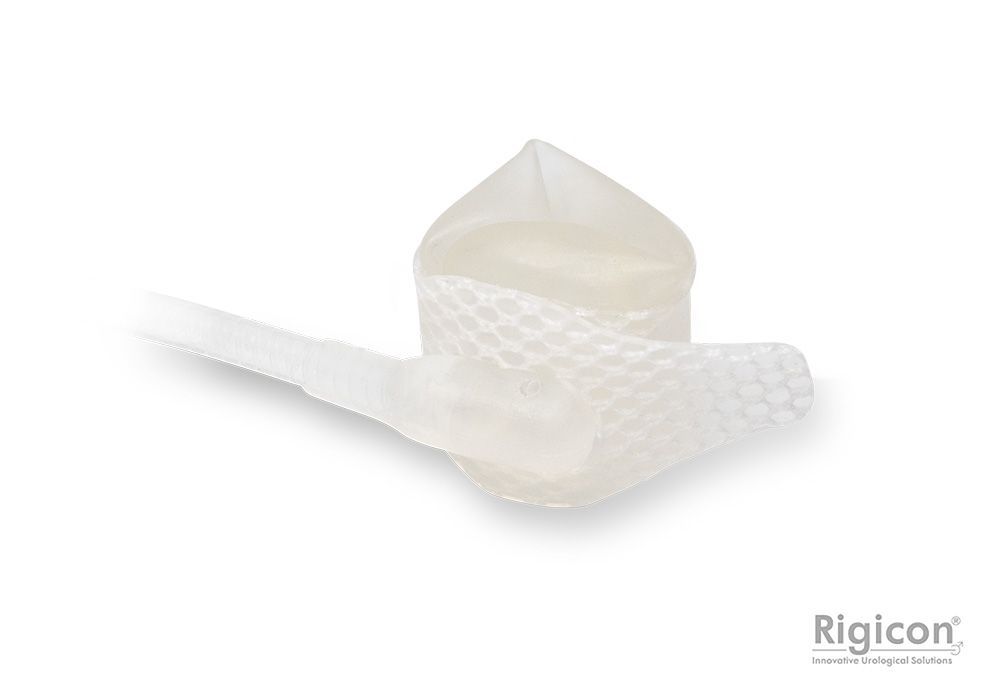
ContiRegular ® Occlusive Cuff
3.5 cm 5.5 cm 9.0 cm 3.75 cm 6.0 cm 9.5 cm 4.0 cm 6.5 cm 10.0 cm 4.25 cm 7.0 cm 11.0 cm 4.5 cm 7.5 cm 12.0 cm 4.75 cm 8.0 cm 13.0 cm
-
ContiRegular ® Occlusive Cuff
- HydroShield™ Coating
Unlike competitors, ContiReflex® is uniquely manufactured with a HydroShield™ hydrophilic coating on all external surfaces, including the pressure-regulating balloon. The HydroShield™ Coating allows for diverse dipping options enabling a customized approach to meet the specific needs of the surgeon and the patient.
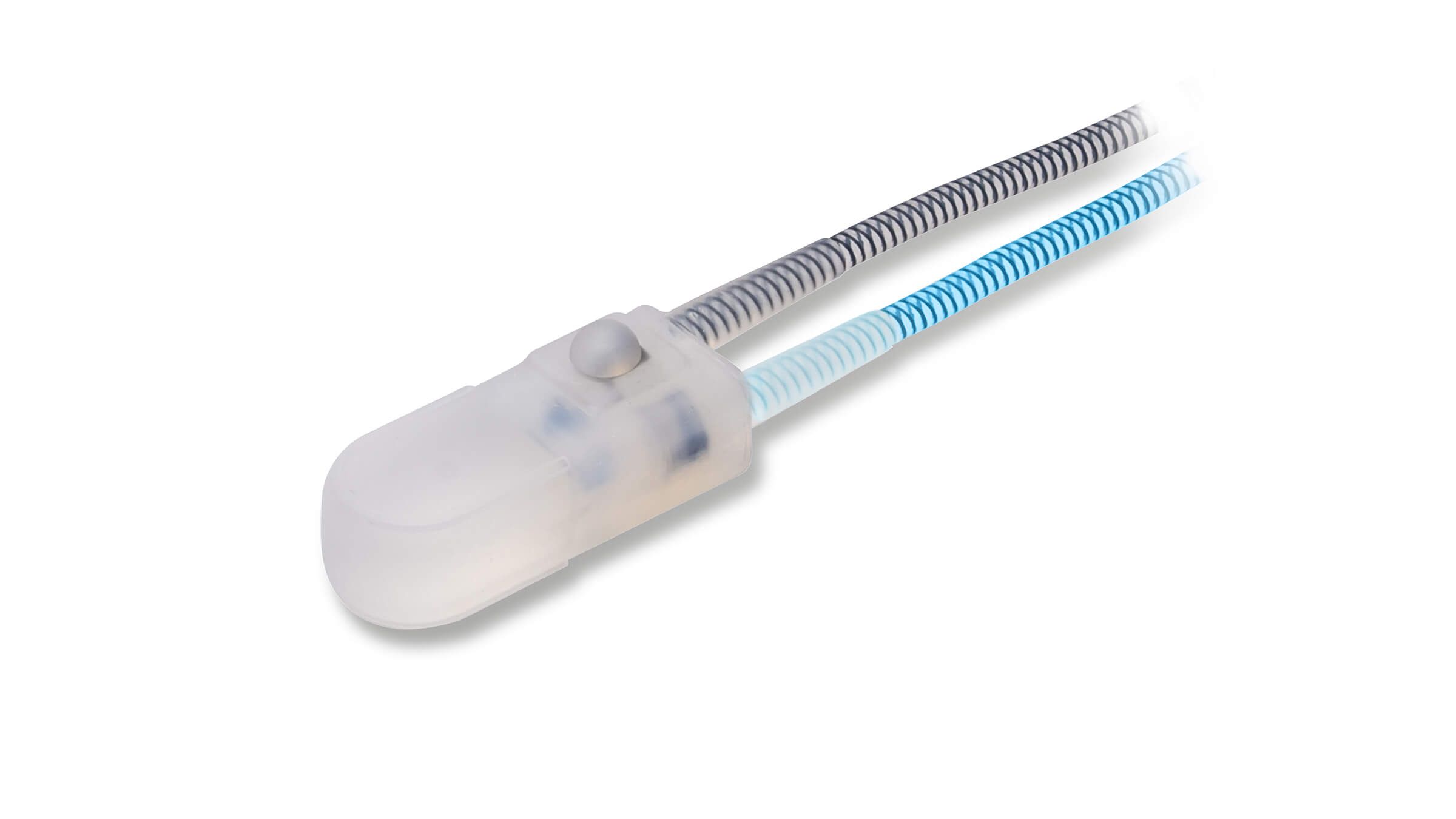
- EasyClick™ Connectors
Kink-resistant tubes are connected easily and manually without the need for any additional assembly tools.
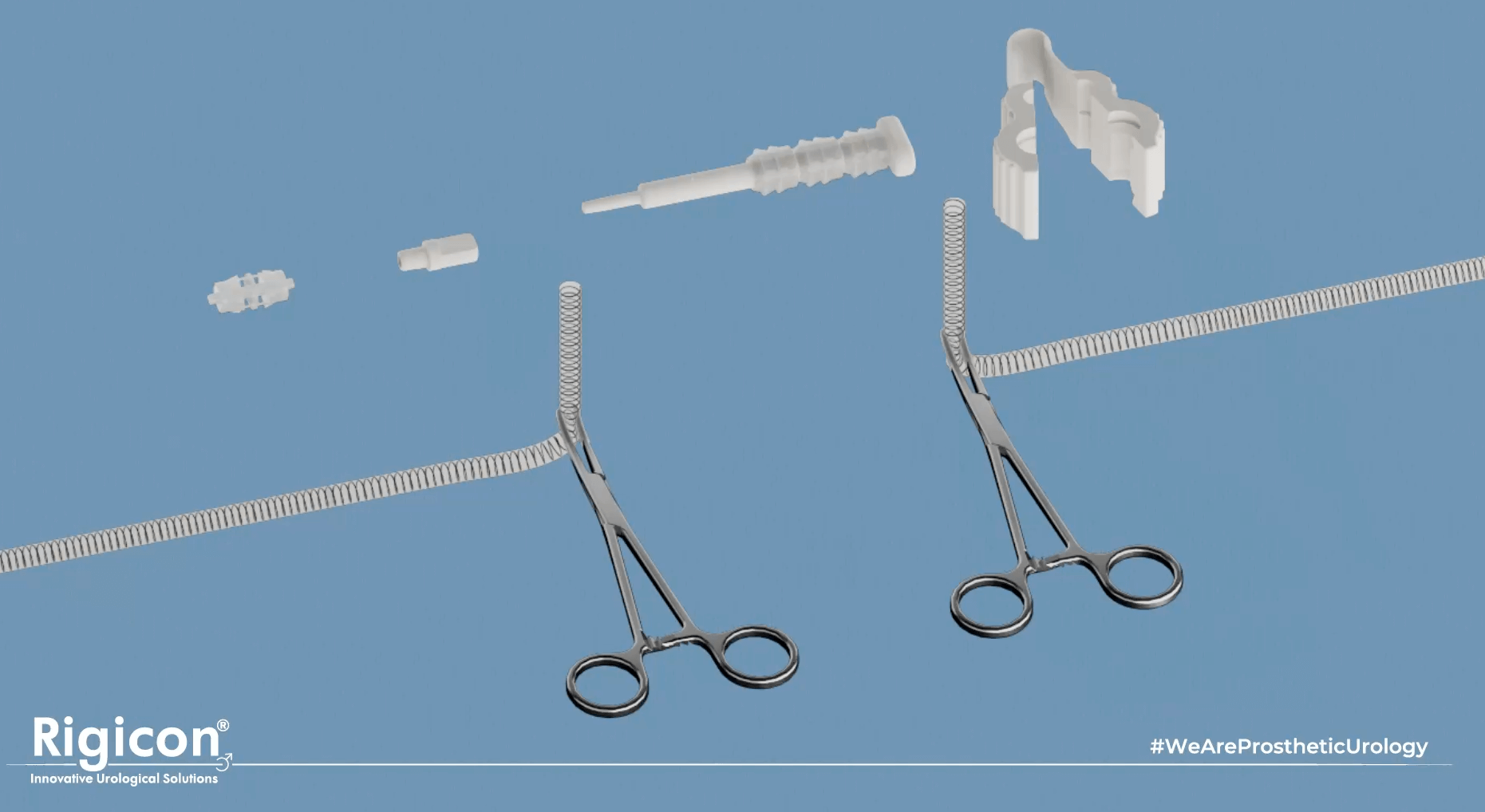
- Rigicon Duality
The ContiReflex® is fully compatible with and can be implanted alongside any of the Infla10® Inflatable Penile Prosthesis models5.
- Conti™ Blue KRT
The Conti™ Artificial Urinary Sphincter Systems feature color-coded kink-resistant tubes, which facilitate reliable intraoperative connections between device components for surgeons. The utilization of blue KRTs, as opposed to the transparent KRTs in legacy systems, enhances visual control to check integrity of the connection.
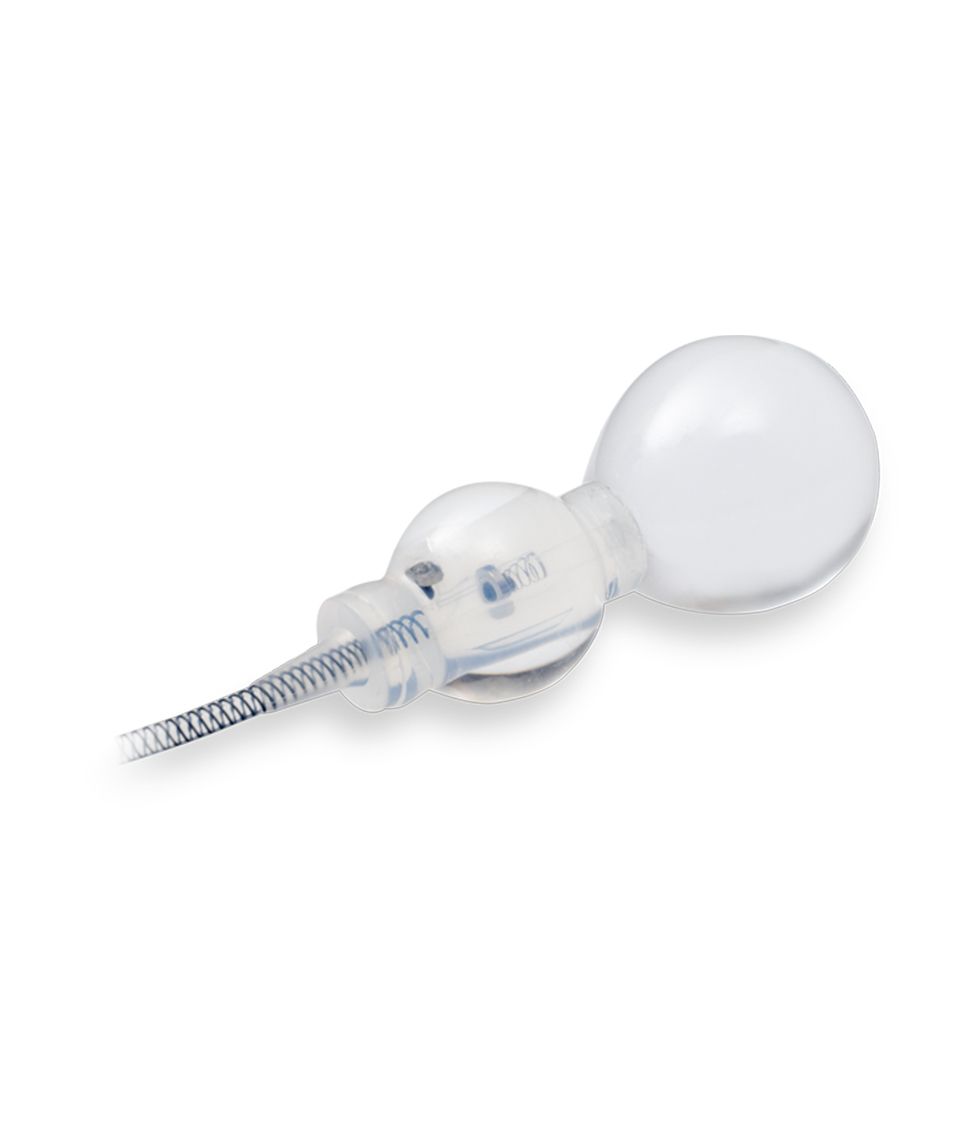
Smart Reflex Balloon senses IAP; changes and modifies occlusive cuff pressure in real-time. Cuff pressure is no longer limited by PRB’s labeled pressure capacity.
- ContiReflex ® PRB Sizes
40 – 49 cm H20 50 – 59 cm H20 60 – 69 cm H20 70 – 79 cm H20 80 – 89 cm H20
- ContiReflex ® PRB Sizes
40 – 49 cm H20 50 – 59 cm H20 60 – 69 cm H20 70 – 79 cm H20 80 – 89 cm H20
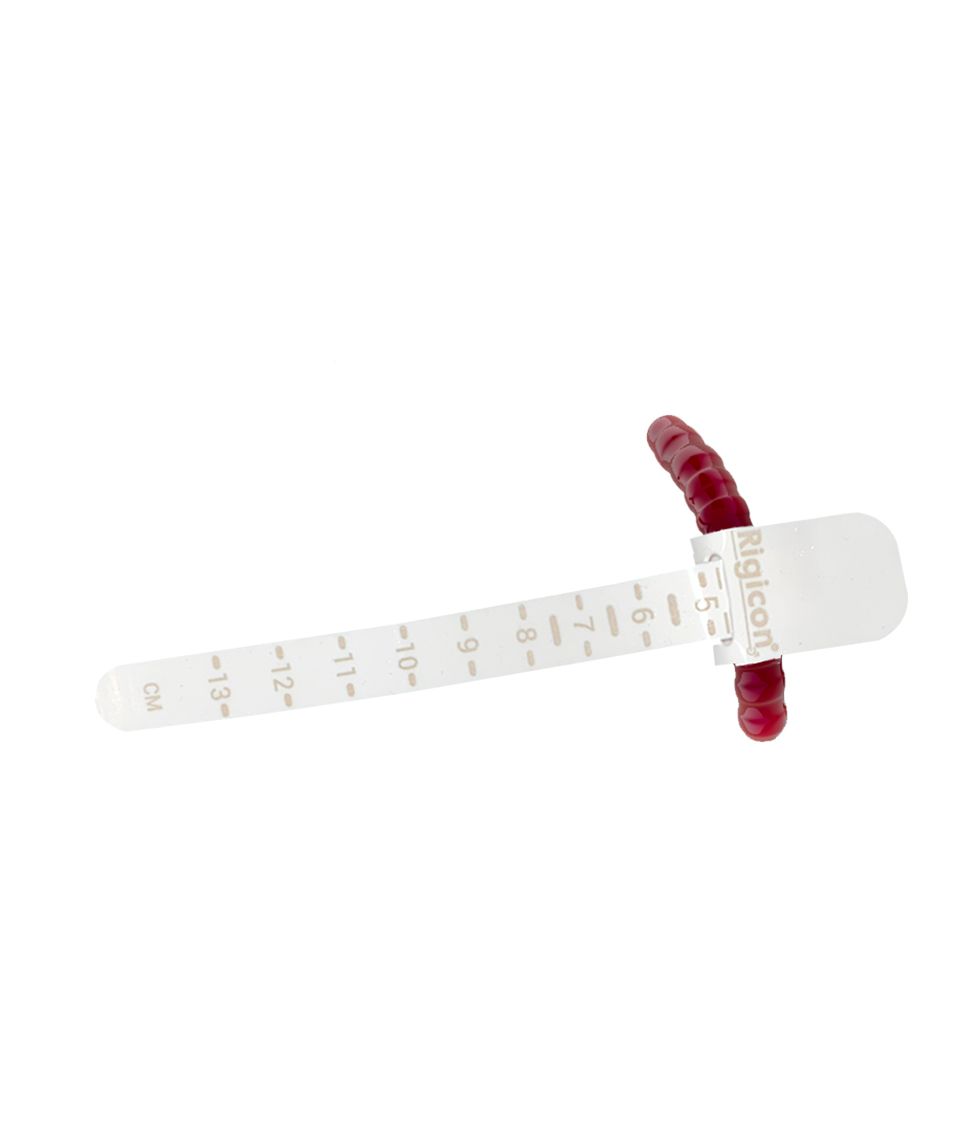
ContiReflex® Enhanced Artificial Urinary Sphincter offers a comprehensive range of occlusive cuff sizes, providing surgeons with unprecedented flexibility in tailoring the device to each patient’s unique urethral anatomy. The diverse selection of cuff sizes spans from 3.5 cm to 13.0 cm, with increments as precise as 0.25 cm in certain ranges, allowing for exceptionally accurate fitting. This extensive array of options enables surgeons to achieve a more personalized and potentially more effective treatment for patients with varying degrees of urinary incontinence.
-
ContiRegular ® Occlusive Cuff
3.5 cm 5.5 cm 9.0 cm 3.75 cm 6.0 cm 9.5 cm 4.0 cm 6.5 cm 10.0 cm 4.25 cm 7.0 cm 11.0 cm 4.5 cm 7.5 cm 12.0 cm 4.75 cm 8.0 cm 13.0 cm
-
ContiRegular ® Occlusive Cuff
3.5 cm 5.5 cm 9.0 cm 3.75 cm 6.0 cm 9.5 cm 4.0 cm 6.5 cm 10.0 cm 4.25 cm 7.0 cm 11.0 cm 4.5 cm 7.5 cm 12.0 cm 4.75 cm 8.0 cm 13.0 cm
Unlike competitors, ContiReflex® is uniquely manufactured with a HydroShield™ hydrophilic coating on all external surfaces, including the pressure-regulating balloon. The HydroShield™ Coating allows for diverse dipping options enabling a customized approach to meet the specific needs of the surgeon and the patient.
Kink-resistant tubes are connected easily and manually without the need for any additional assembly tools.
The ContiReflex® is fully compatible with and can be implanted alongside any of the Infla10® Inflatable Penile Prosthesis models5.
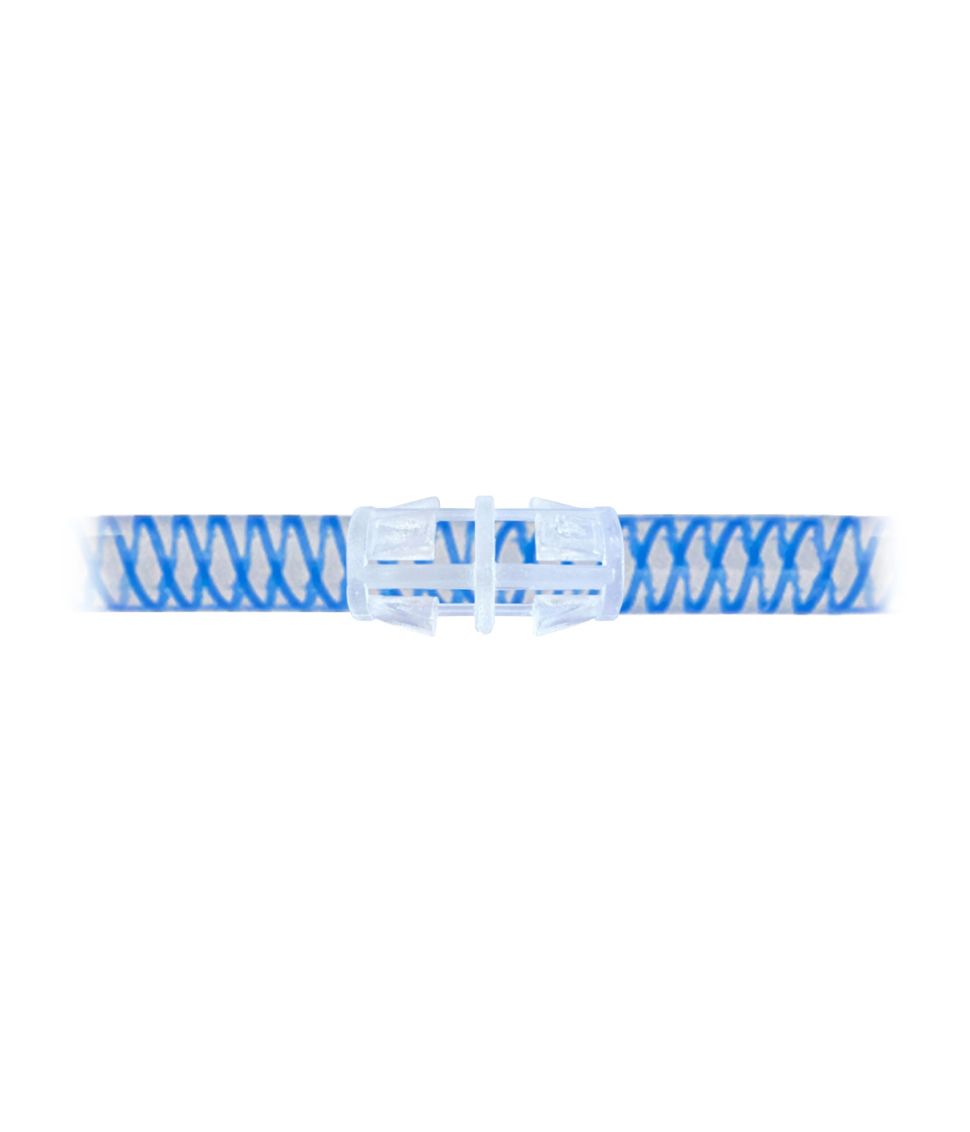
The Conti™ Artificial Urinary Sphincter Systems feature color-coded kink-resistant tubes, which facilitate reliable intraoperative connections between device components for surgeons. The utilization of blue KRTs, as opposed to the transparent KRTs in legacy systems, enhances visual control to check integrity of the connection.
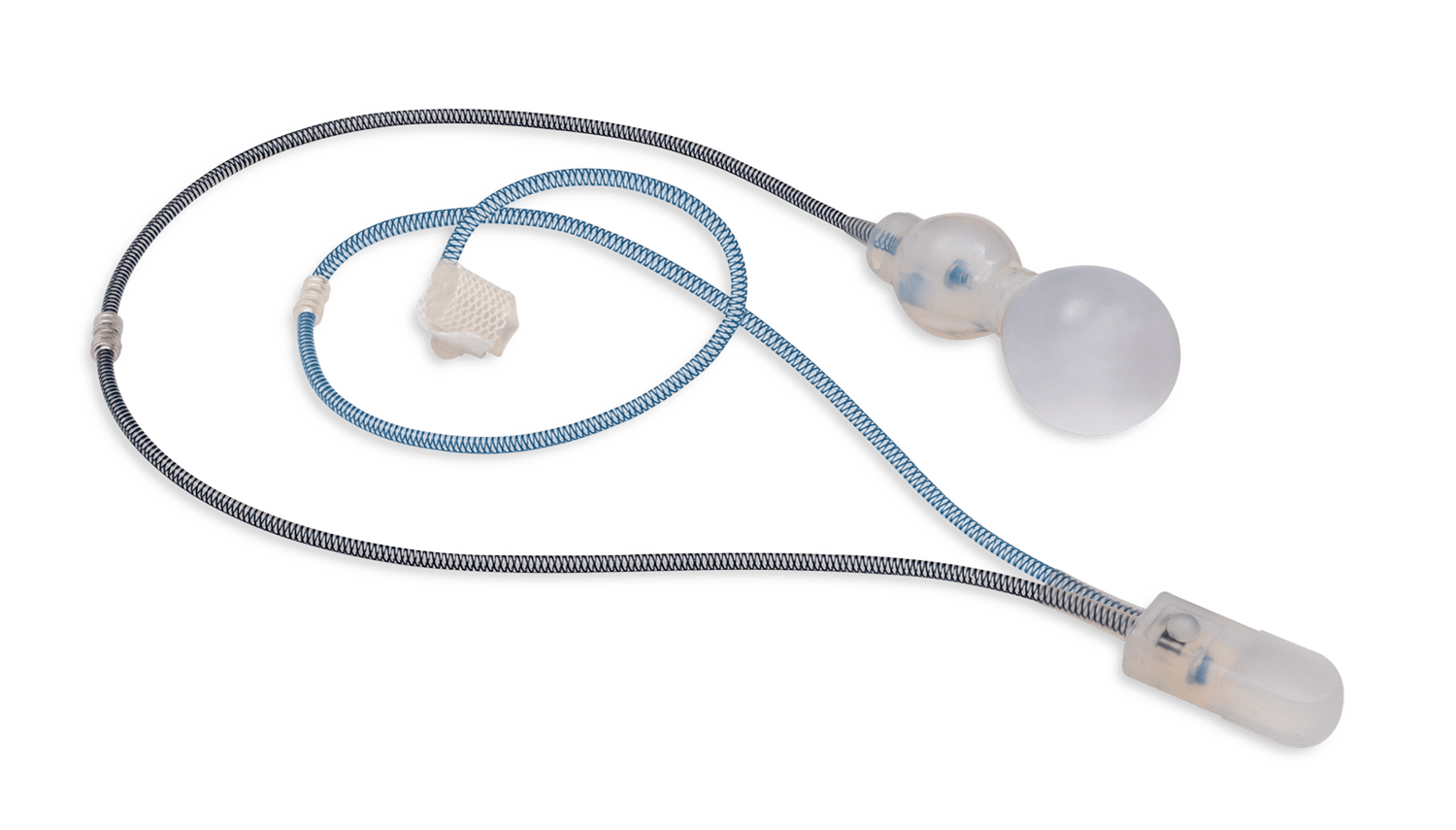

Smart Reflex Balloon
Occlusive Cuff
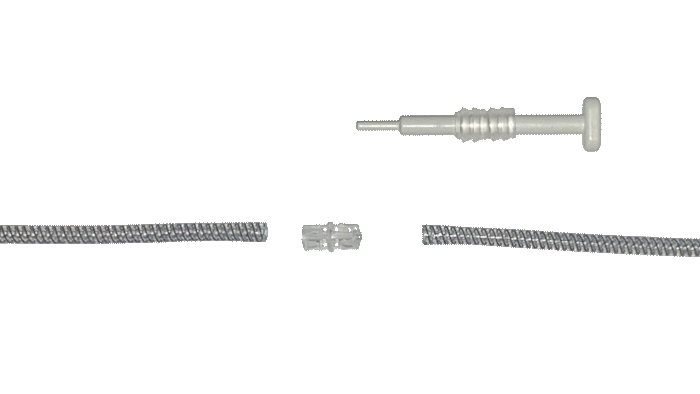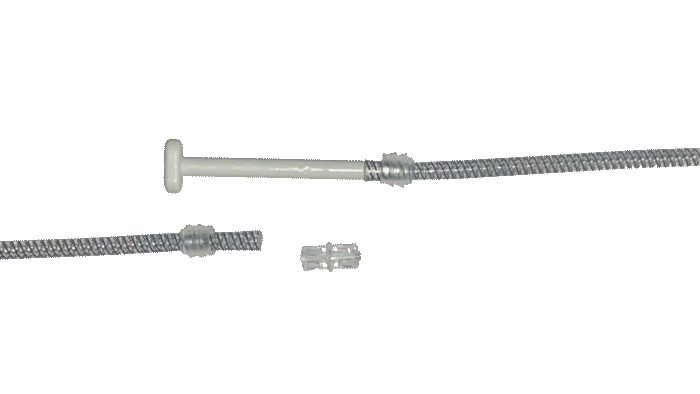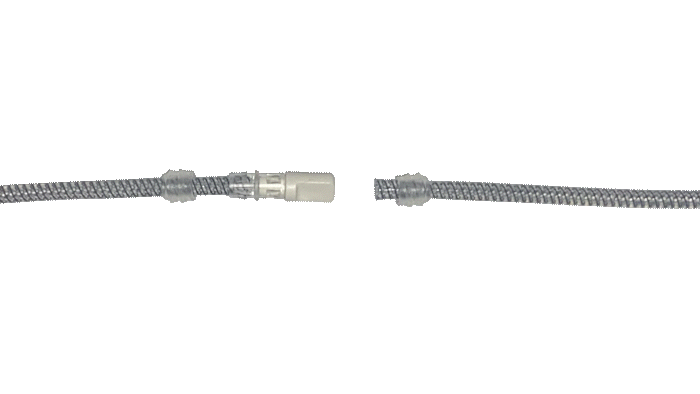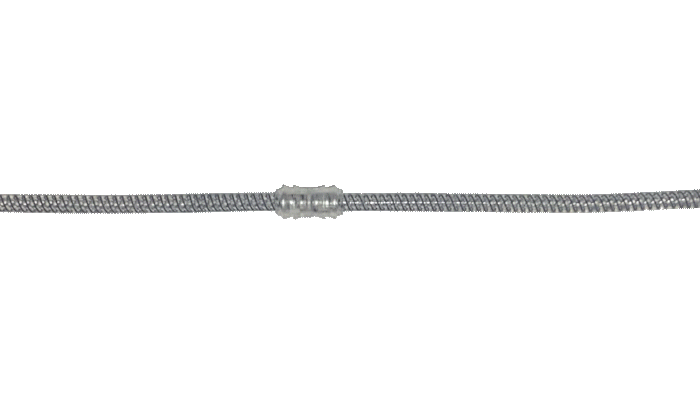
EasyClick™ Connector

Kink Resistant Tubing

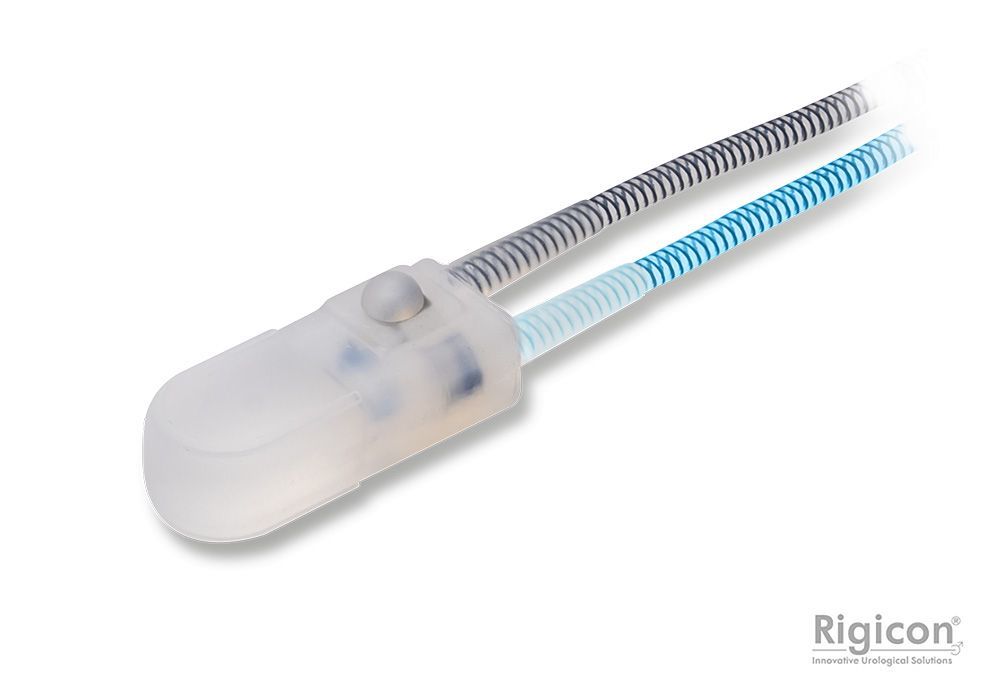
Deactivation Button

Control Pump
- How to use your ContiReflex® Artificial Urinary Sphincter?Caution: Professional Medical Content
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
- ContiReflex® Artificial Urinary Sphincter Implantation | Penoscrotal Approach | Prof. van RenterghemCaution: Professional Medical Content
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
- Rigicon Talks – Session 2 – Top 10 Questions Answered about Artificial Urinary Sphincters
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
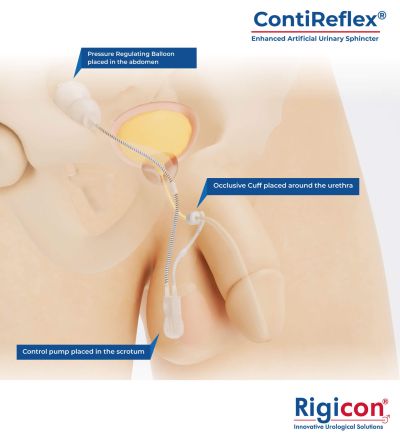
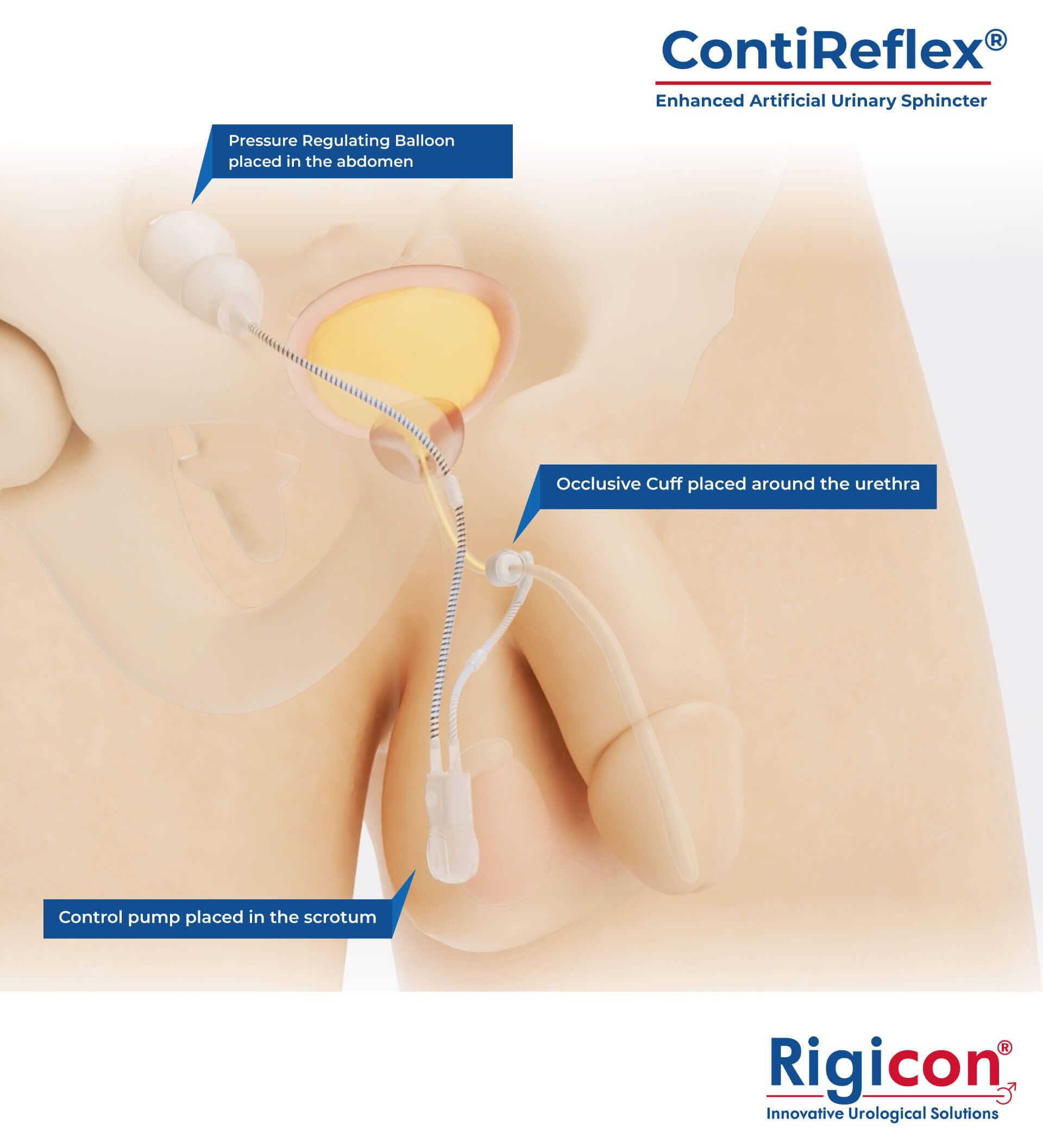
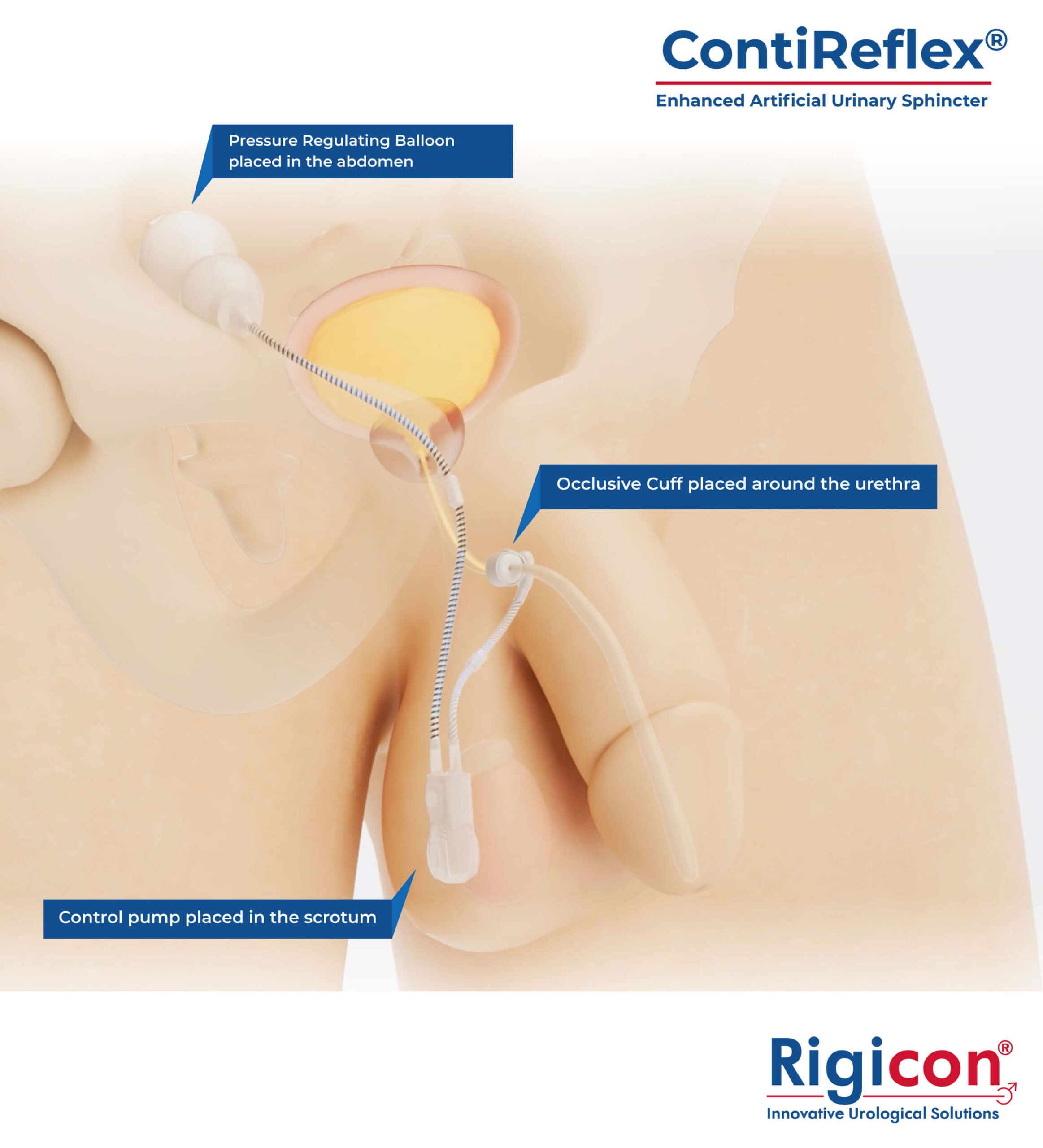
- ContiReflex® Enhanced Artificial Urinary Sphincter Product Illustration
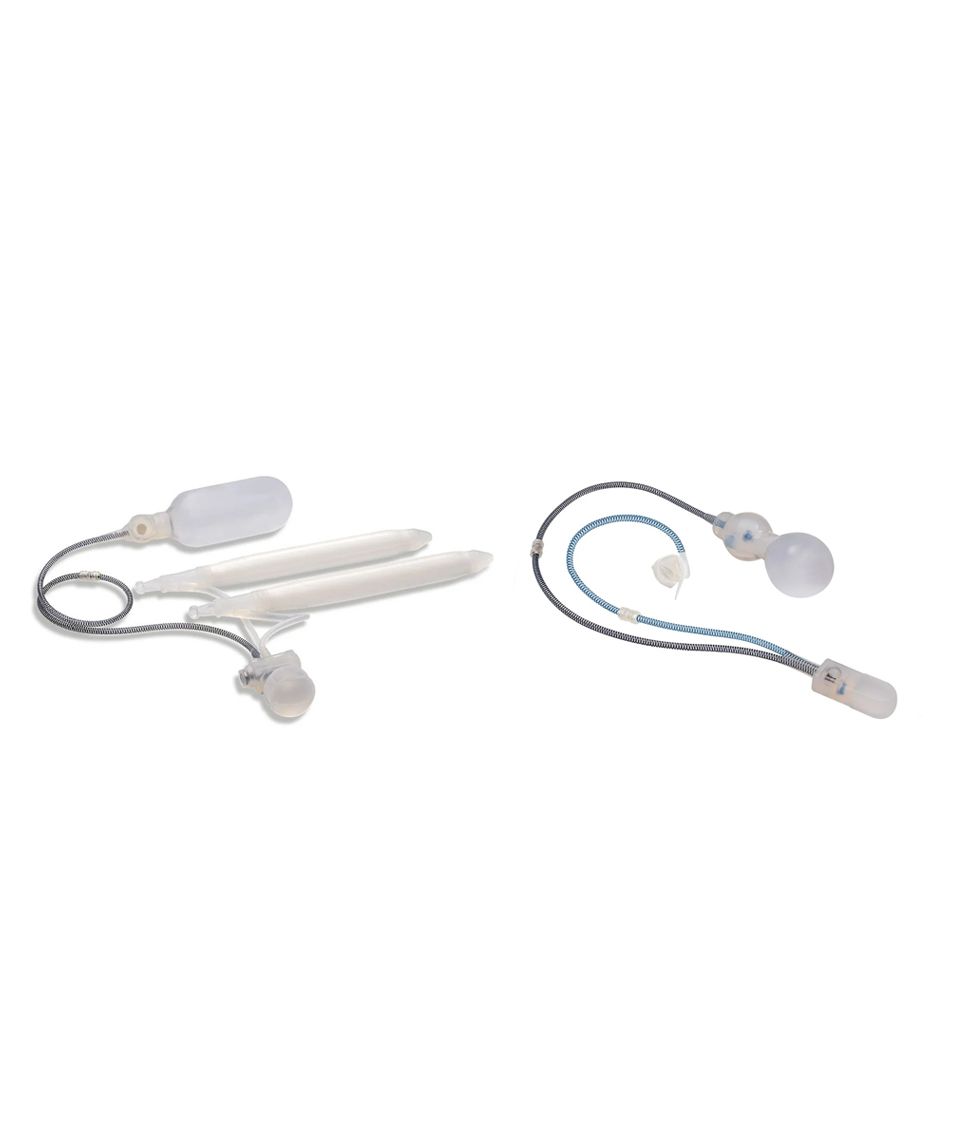
The ContiReflex® system is designed to help patients regain control of urinary function. It consists of three key components: a smart reflex balloon placed in the abdomen, an occlusive cuff positioned around the urethra, and a control pump located in the scrotum. When the control pump is activated, the cuff tightens around the urethra to prevent urine leakage. To urinate, the patient squeezes the control pump, which temporarily loosens the cuff, allowing urine to pass. This system is an effective solution for patients suffering from urinary incontinence, providing a discreet and reliable method for managing bladder control.
The ContiReflex® system is designed to help patients regain control of urinary function. It consists of three key components: a smart reflex balloon placed in the abdomen, an occlusive cuff positioned around the urethra, and a control pump located in the scrotum. When the control pump is activated, the cuff tightens around the urethra to prevent urine leakage. To urinate, the patient squeezes the control pump, which temporarily loosens the cuff, allowing urine to pass. This system is an effective solution for patients suffering from urinary incontinence, providing a discreet and reliable method for managing bladder control.
Conti™ Artificial Urinary Sphincter
Product Code | ContiRegular® Occlusive Cuff |
|---|---|
| CTR-OC35 | 3.5 cm |
| CTR-OC375 | 3.75 cm |
| CTR-OC40 | 4.0 cm |
| CTR-OC425 | 4.25 cm |
| CTR-OC45 | 4.5 cm |
| CTR-OC475 | 4.75 cm |
| CTR-OC50 | 5.0 cm |
| CTR-OC55 | 5.5 cm |
| CTR-OC60 | 6.0 cm |
| CTR-OC65 | 6.5 cm |
| CTR-OC70 | 7.0 cm |
| CTR-OC75 | 7.5 cm |
| CTR-OC80 | 8.0 cm |
| CTR-OC85 | 8.5 cm |
| CTR-OC90 | 9.0 cm |
| CTR-OC95 | 9.5 cm |
| CTR-OC100 | 10.0 cm |
| CTR-OC110 | 11.0 cm |
| CTR-OC120 | 12.0 cm |
| CTR-OC130 | 13.0 cm |
Product Code | Accessory Kit |
|---|---|
| CT-AK | Conti™ Accessory Kit |
How does the Smart Reflex Balloon in ContiReflex® enhance the treatment of stress urinary incontinence?
The unique Smart Reflex Balloon in ContiReflex® is designed to sense changes in intraabdominal pressure and adapt the pressure on the urethra accordingly. This dynamic adjustment potentially offers improved patient outcomes with stress urinary incontinence, particularly during activities that increase abdominal pressure, like coughing or exercising. It ensures that cuff occlusion pressure is responsive to bodily movements, providing reliable continence control.
Can the ContiReflex® Artificial Urinary Sphincter be used in combination with treatments for erectile dysfunction?
Yes, ContiReflex® is compatible with the Infla10® Inflatable Penile Prosthesis, allowing for concurrent management of stress urinary incontinence and erectile dysfunction. This compatibility is particularly beneficial for patients who have undergone prostate surgery and are experiencing both conditions. The integration of these devices provides a comprehensive approach to post-prostatectomy rehabilitation.
What can I expect in terms of recovery after getting the ContiReflex® Artificial Urinary Sphincter? When can I return to normal activities?
The recovery period following the implantation of ContiReflex® typically lasts up to 6 weeks, but this can vary depending on individual circumstances. During the first 4-6 weeks post-surgery, we generally advise patients to avoid demanding activities. Your physician will activate the device at a follow-up appointment within this period. You’ll receive specific guidance for your recovery, but most patients gradually resume their normal activities, ensuring a comfortable and successful recovery process.
Is the ContiReflex® device noticeable? Will others be able to tell that I have it implanted?
No, the ContiReflex® is designed to be completely discreet. All of its components are internally implanted and fully concealed within your body. There are no visible signs of the device externally, so your privacy and comfort are maintained. Rest assured that the device is designed to be discreet and unnoticeable to others, allowing you to confidently engage in your daily activities.
I’m concerned about the ease of using the ContiReflex® device. How simple is it to operate the control pump?
The ContiReflex® is designed with ease of use in mind, particularly when it comes to the control pump. This pump is engineered to be very user-friendly, allowing easy manipulation. You’ll be able to manage the device with minimal effort, providing control over urinary continence without significant physical strain or complexity. Your physician will guide you on how to use the pump effectively, ensuring you feel confident in managing the device independently.
Are there any unique features of the ContiReflex® that make it stand out compared to other artificial urinary sphincters?
Yes, the ContiReflex® has several unique features. Its innovative Reflex Balloon adapts to changes in intra-abdominal pressure, providing real-time adjustment for potentially better continence control. Additionally, the device offers a range of cuff sizes for a precise fit and a hydrophilic coating compatible with various antibiotics, enhancing surgical flexibility and patient-specific care. These features collectively contribute to the device’s effectiveness and patient comfort, making it a distinguished choice in the treatment of stress urinary incontinence.
As someone who’s active and enjoys sports, how will the ContiReflex® affect my lifestyle? Can I continue with activities like gym workouts or running?
The ContiReflex® is designed to adapt to changes in intraabdominal pressure, making it suitable for active lifestyles. After the initial recovery period, nearly all patients can return to their regular physical activities, including gym workouts and running. The device’s ability to respond to pressure changes means it provides protection during various movements. However, we recommend discussing your specific activity level with your physician to get personalized advice on resuming such activities post-implantation.
Will I need to make any long-term adjustments or lifestyle changes after the ContiReflex® implantation?
While ContiReflex® is designed to enhance your quality of life and provide better control over urinary incontinence, some lifestyle adjustments may be beneficial post-implantation. These might include managing fluid intake, and avoiding excessive demanding activities, and regular follow-up appointments to ensure the device is functioning optimally. Your physician will provide detailed guidance based on your individual situation to ensure the best outcomes with your ContiReflex®.
I’ve had urinary incontinence for a while. How effective is the ContiReflex® in managing long-term incontinence issues?
Artificial Urinary Sphincter systems are proven to be highly effective for long-term management of stress urinary incontinence, especially in cases arising from intrinsic sphincter deficiency, such as after prostate surgery. ContiReflex®’s innovative design, including the Reflex Balloon, provides dynamic pressure adjustment, enhancing continence control.
Are there any specific considerations or care routines I should follow to maintain the ContiReflex® device’s effectiveness over time?
To maintain the effectiveness of your ContiReflex®, it’s important to adhere to the care routines advised by your physician. This includes regular check-ups to monitor the device’s function, proper handling of the control pump, and being mindful of activities that could impact the device. Additionally, maintaining a healthy lifestyle, managing fluid intake, and avoiding activities that exert excessive pressure on the abdominal area can contribute to the long-term success of the device.
FAQ’s Disclaimer
The content is not intended to be a substitute for, nor does it replace professional medical advice, diagnosis, or treatment. If you have any concerns or questions about your health, you should always consult with a physician or other health-care professional.
-
Federal law (USA) restricts this device to sale by or on the order of a physician.
-
Not available for sale within the United States.