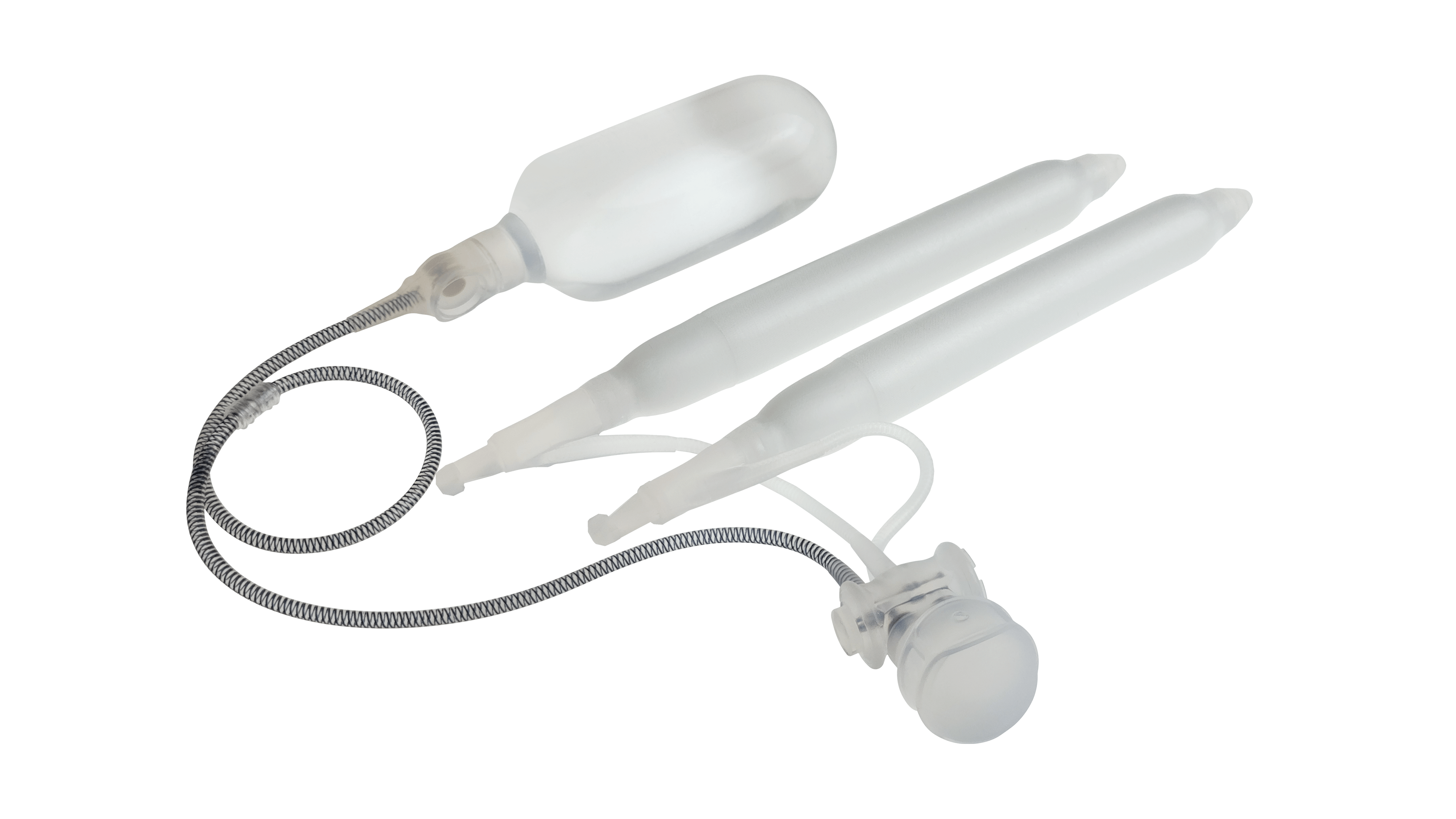
Infla10® Pulse™ Series
Dynamic Inflatable Penile Prosthesis (DIPP)
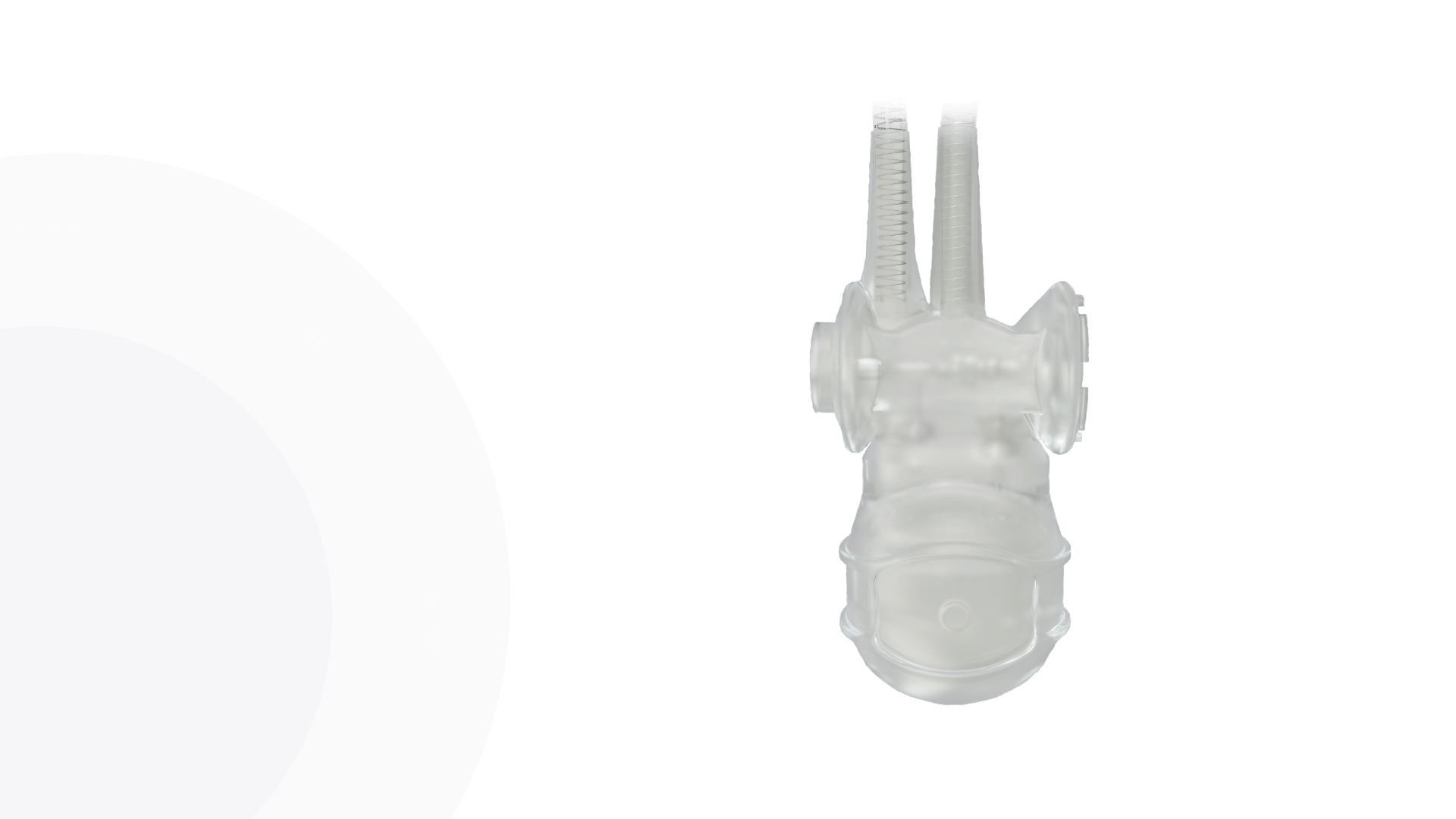
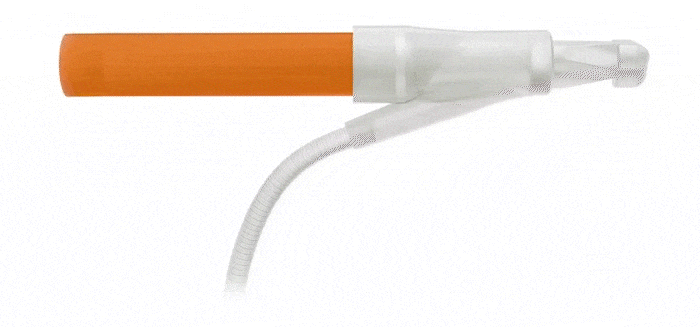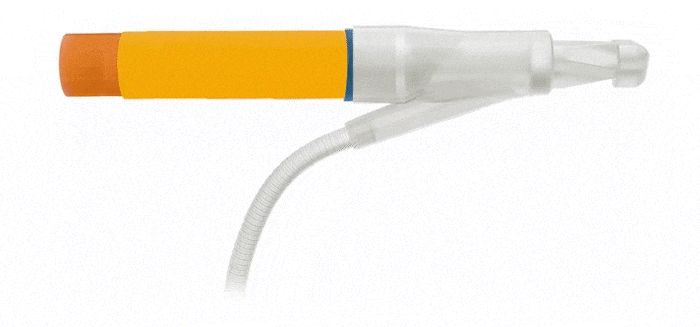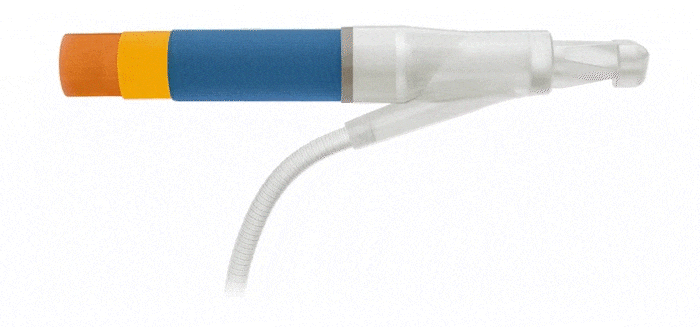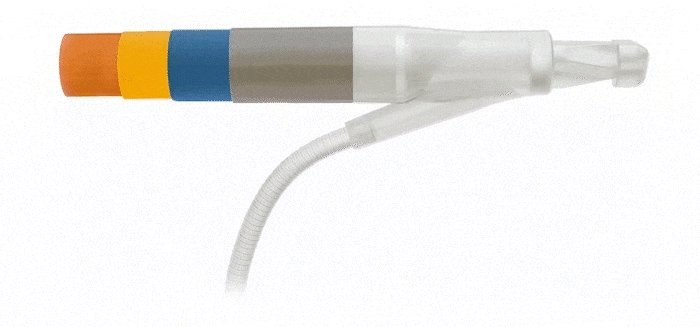
Pulse™ Pump
Pressure Valve
Infla10® Pulse™
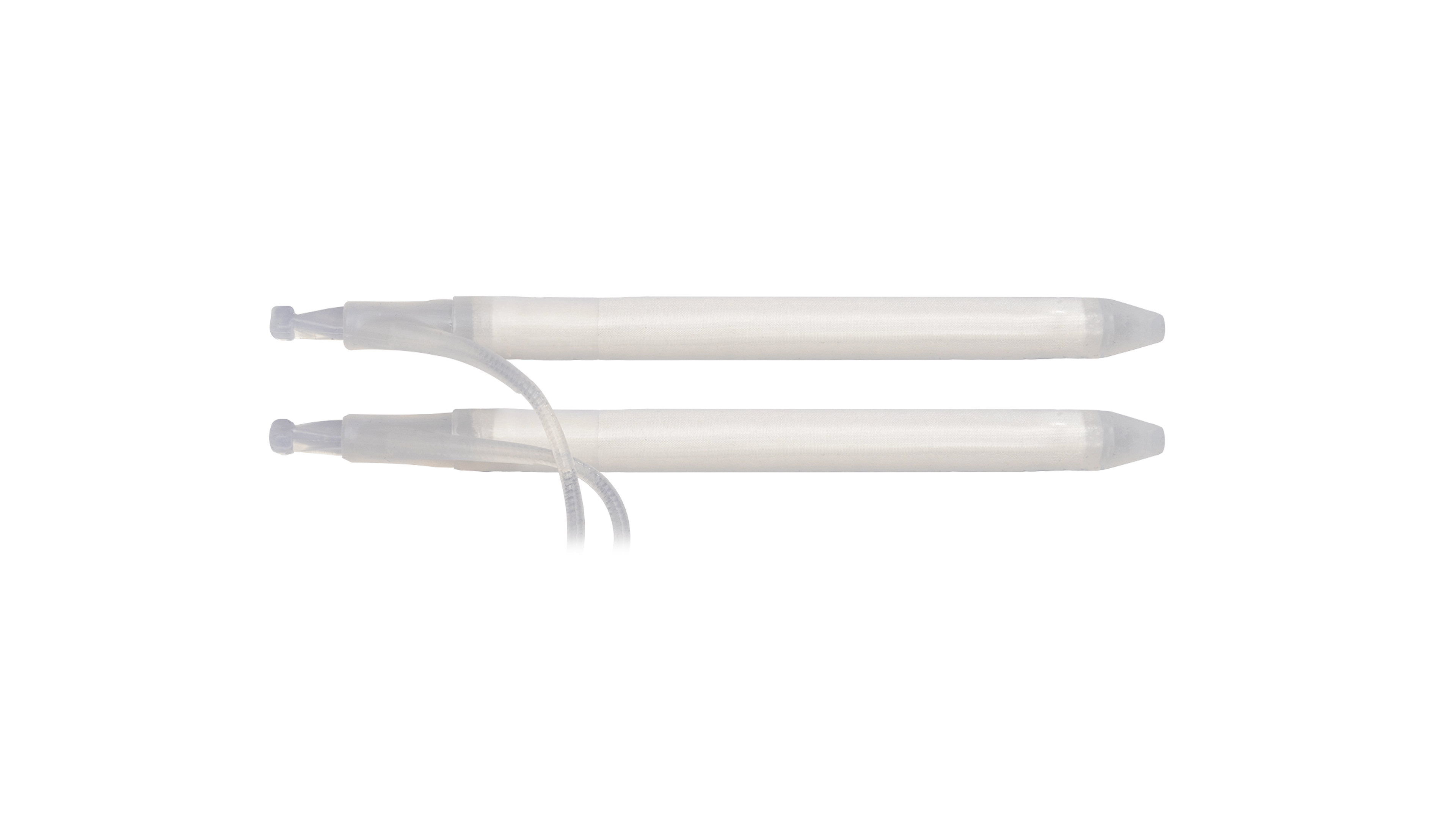
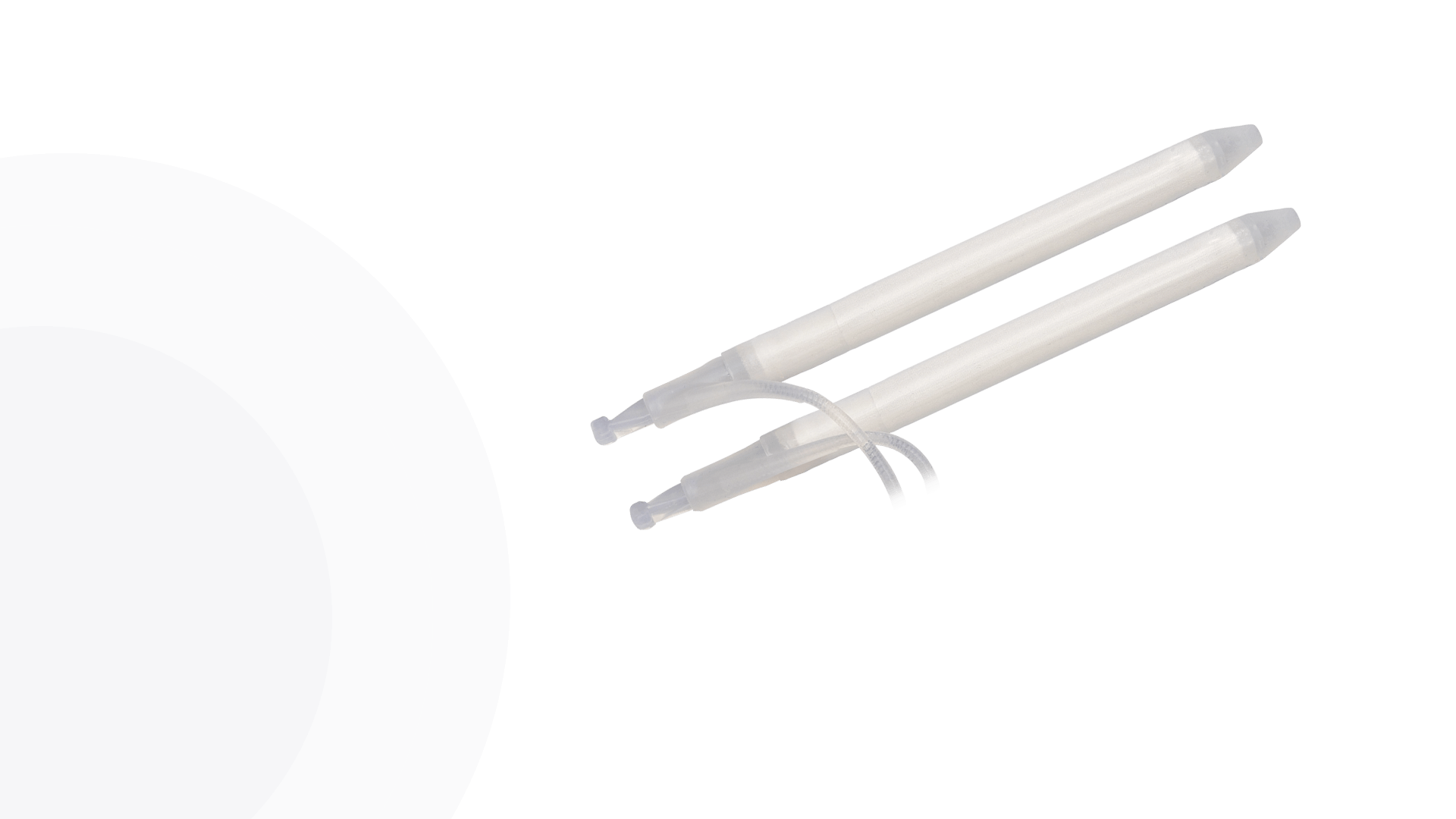
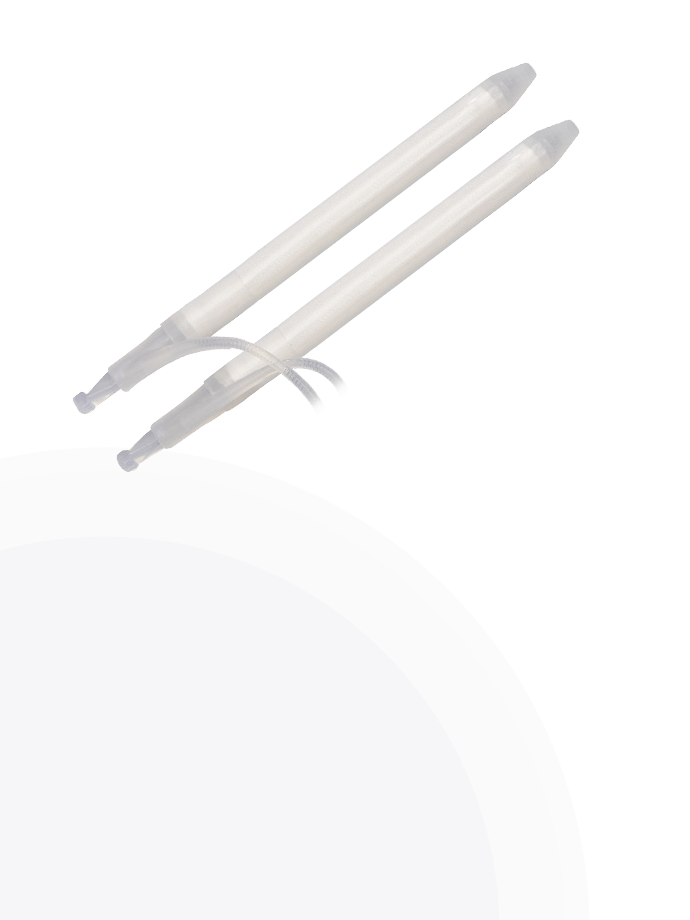
Cylinders
AdaptiveReservoir ™

Cylinders
Product Details
Rigicon offers the Infla10® Pulse™ three-piece dynamic inflatable penile prostheses, designed for men suffering from chronic, organic erectile dysfunction (ED) and suitable candidates for penile prosthesis implantation.
Engineered for durability, great rigidity, and a natural feel, Infla10® Pulse™ is developed with advanced urological expertise, focusing on delivering a safe and effective solution for both patients and healthcare providers.
| Feature | Pulse™ X | Pulse™ AX | Pulse™ |
|---|---|---|---|
|
Girth Expansion |
Up to 46 mm |
Up to 46 mm |
Yes |
|
Length Expansion |
– |
Up to 25%3 |
– |
|
Narrower Cylinders |
– |
– |
Yes |
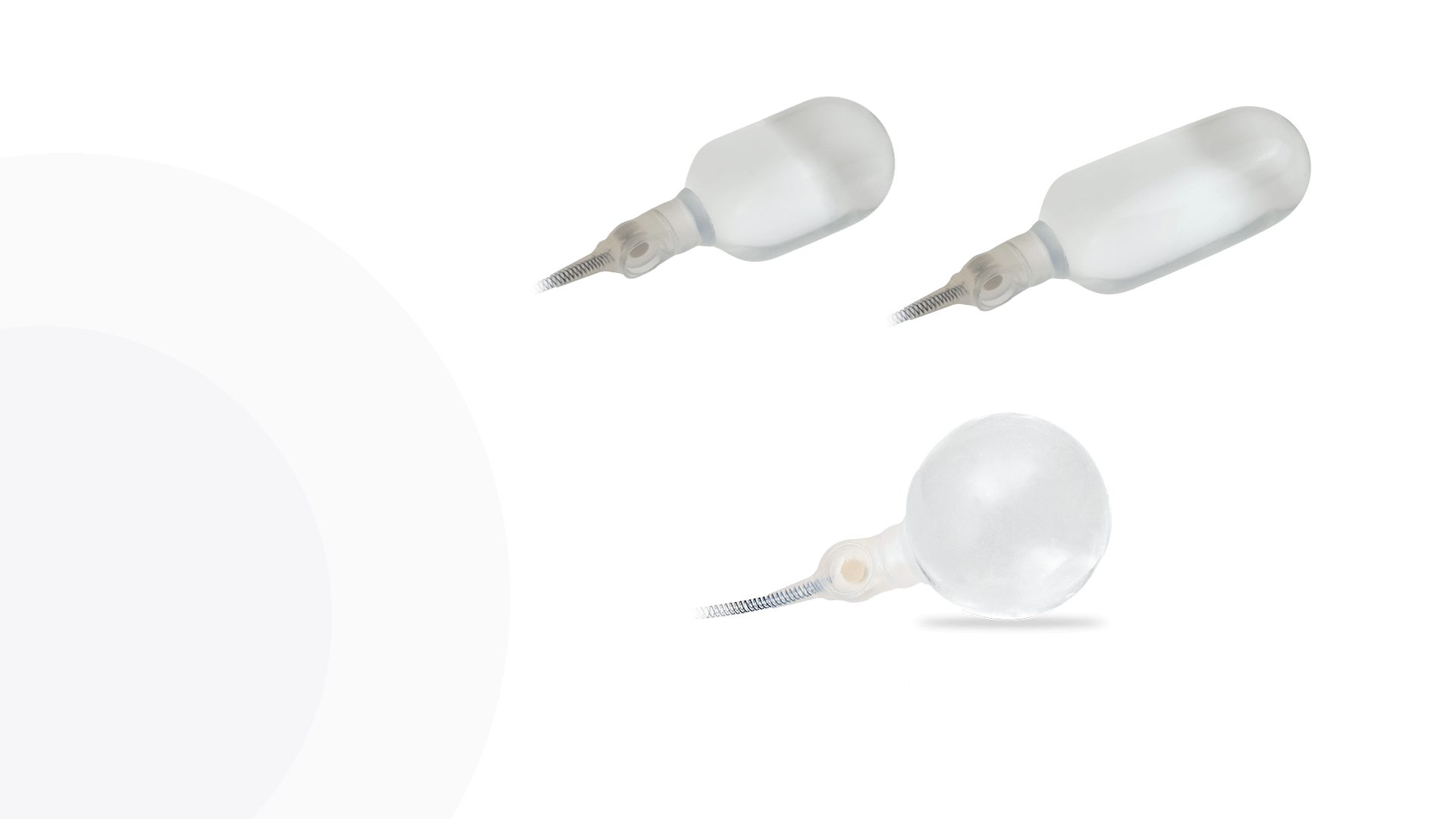
Infla10® Pulse™ X and AX achieve significant girth expansion capabilities of up to 46 mm, ensuring optimal patient satisfaction and anatomical fit.
Infla10® Pulse™ AX offers exceptional length expansion of up to 25% relative to the original length, depending on the patient’s anatomy3.
Infla10® Pulse™ combines advanced girth expansion with optimized design options for a wide range of patients, including those requiring narrower cylinders or with scarred corpora.
What is DIPP?
The Dynamic Inflatable Penile Prosthesis (DIPP) represents the latest generation in penile prosthesis technology, offering significant advancements over traditional inflatable penile prostheses (IPPs). Designed to enhance both function and comfort, DIPP provides a more natural and satisfying experience for users.
Key Features of DIPP:
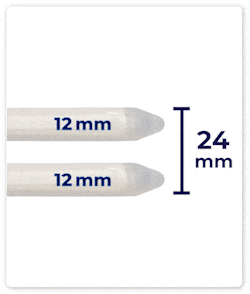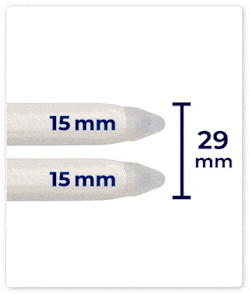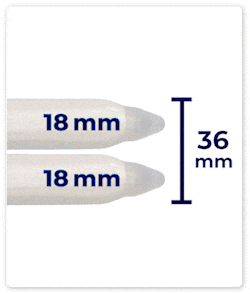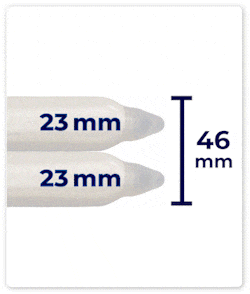
Up to 46 mm Expansion: DIPP’s cylinders can expand up to 46 mm in girth, providing a fuller and more natural feel.
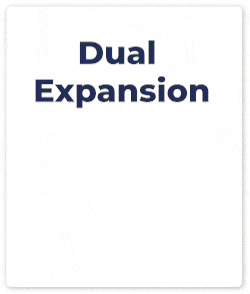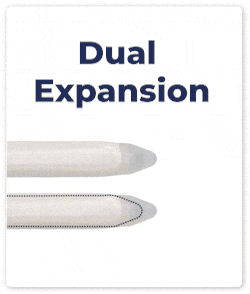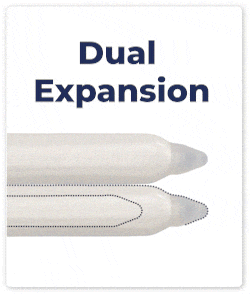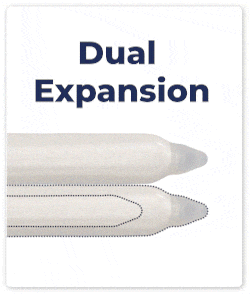
Dual Expansion: Both cylinders expand simultaneously in length and girth, ensuring consistent and proportional enhancement.
Strong Column Strength: Experience a firm and natural-feeling erection without compromising rigidity in the erect state.
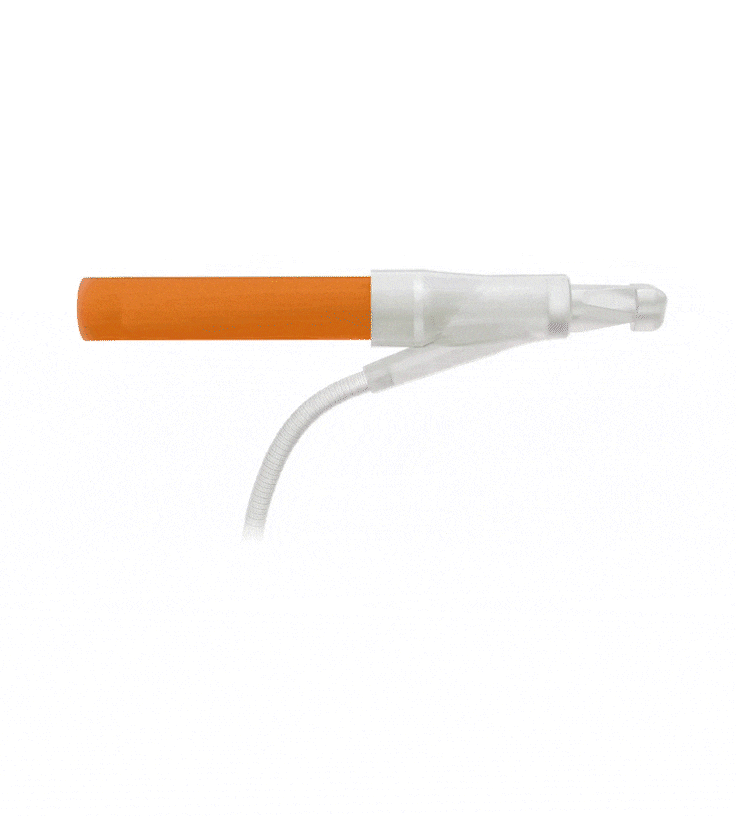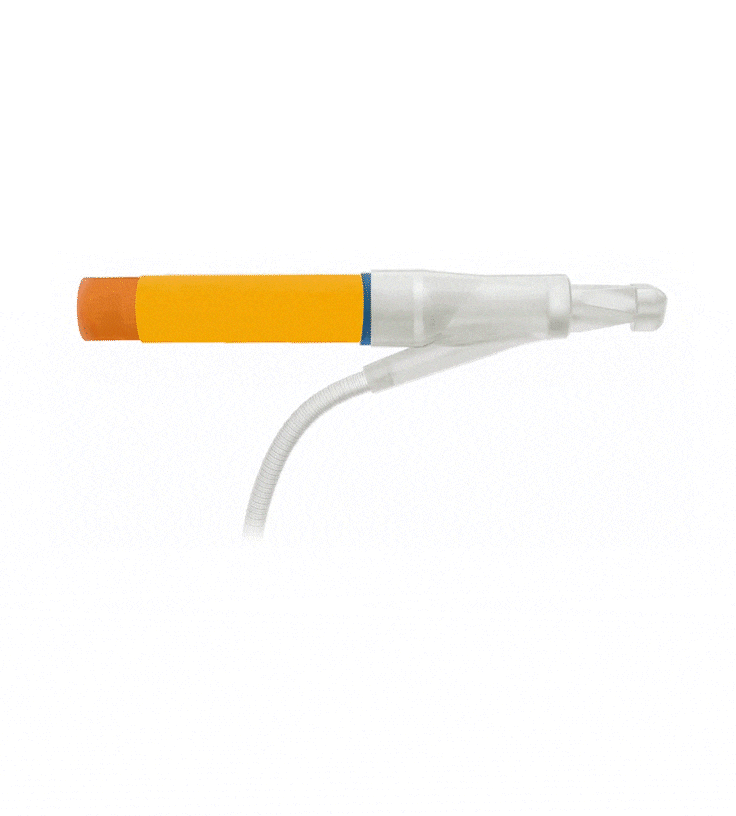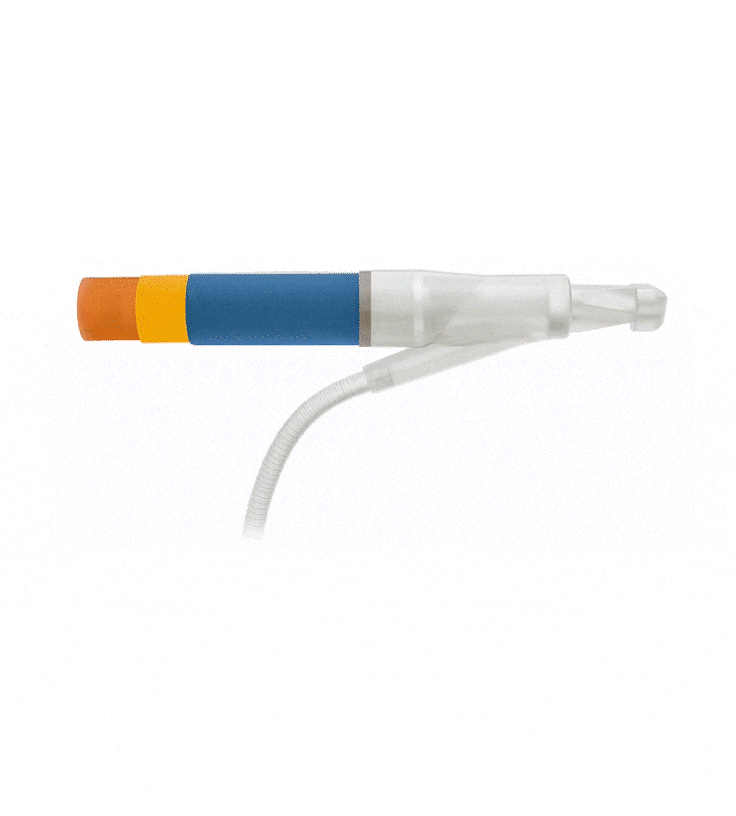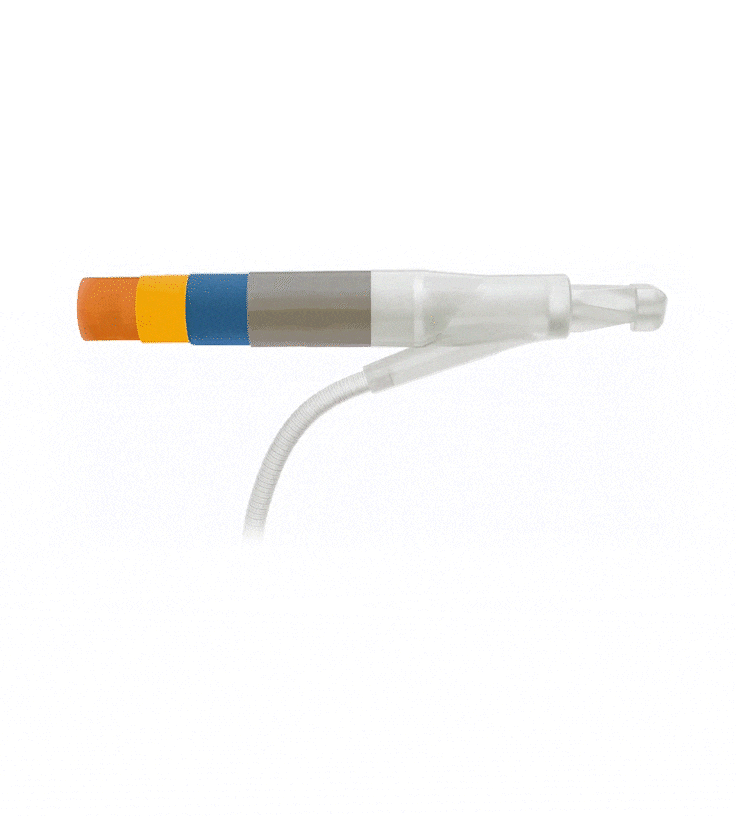
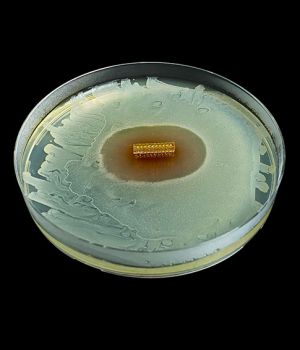
Reinforced Cylinders: Infla10®️ cylinders are reinforced with a novel fourth layer, increasing surface integrity and offering a potential solution to cylinder surface erosion—one of the main causes of device malfunction.
Ideal for Smaller Sizes: DIPP is the best solution for individuals seeking significant girth and length enhancement, even if starting from a smaller size.

Effortless Inflation and Deflation: Engineered for efficient performance, the ergonomically optimized Pulse™️ pump features an innovative design that prioritizes ease of identification and effortless grasp.
By combining a natural feel that simulates bodily functions for an authentic experience, precision control allowing precise adjustments in size and firmness, efficient operation enhancing inflation and deflation mechanisms, and a user-friendly, ergonomically improved design for ease of use, Pulsametric technology™ delivers unparalleled performance.
Why Choose DIPP?
DIPP brings a dynamic approach to penile prosthesis, focusing on:
Natural Experience: Mimics the body’s natural processes for a more authentic feel.
Advanced Technology: Incorporates cutting-edge materials and design for improved performance.
Enhanced Confidence: Provides improvements in size and rigidity, contributing to overall satisfaction.
- Wilson SK, Rossello M, Maria P, Wen L, Carrion R, Perito P, Ralph D, Antonini G, Caravajal A, Chung E. Initial safety outcomes for Rigicon Infla10®️ inflatable penile prosthesis. British Journal of Urology epub ahead of print 1/2023
- Wilson SK, Haximolla H, Kua B, Testa G, Love C, Chung E, Rossello M, Carvajal A. Survival from revision surgery for new Rigicon Inla10®️ 3-piece prosthesis is comparable to competitive devices. Urology;180:257-61
- Wilson SK, Chung E, Langford B, et al. First safety outcomes for Rigicon ContiClassic®️ Artificial Urinary Sphincter. Int J of Impot Res. 7/2023 epub ahead of print
- Atwater B, Kirkuk D, Wilson SK, Koka O, Purohit R, Muñoz V, Dunglison, Gross M. Shor-term revision rate for Rigicon Testi10TM in adolescents and adults. A Retrospective Review. Int J Impot Res. 3/2024 epub ahead of print.
- Wilson SK, Chung E, Langford B, Rees Rowland, Schlessinger R, Koufogiannis G, Van Renterghem K. Early safety outcomes of ContiReflex AUS for treating male incontinence. Presented as moderated poster at 2024 AUA
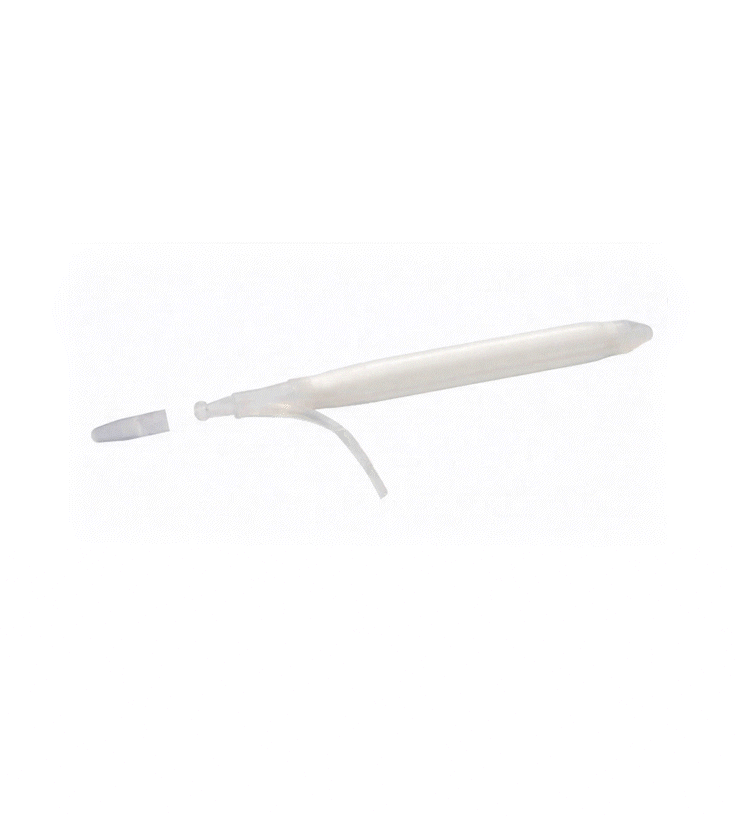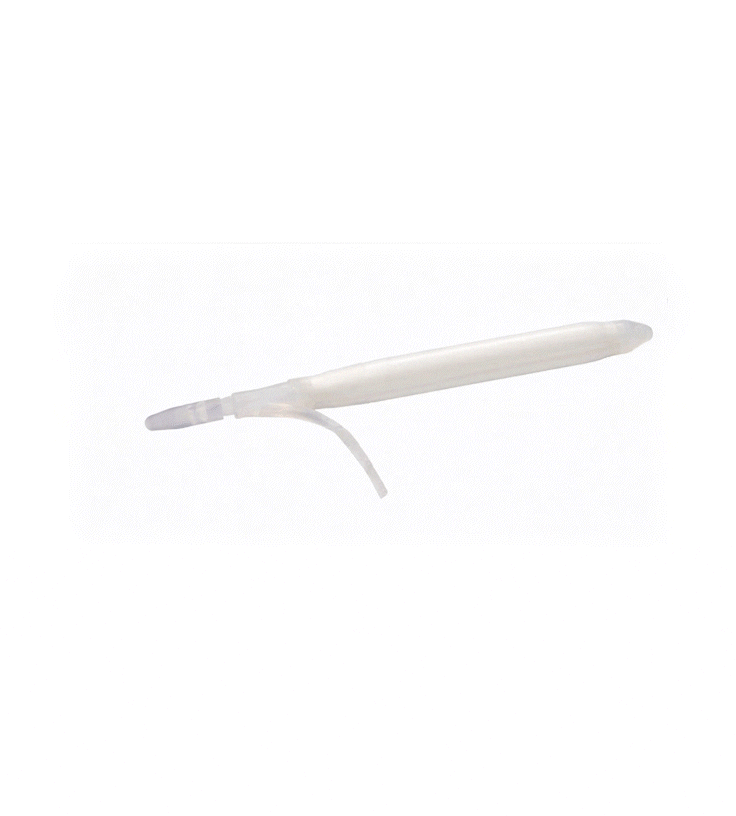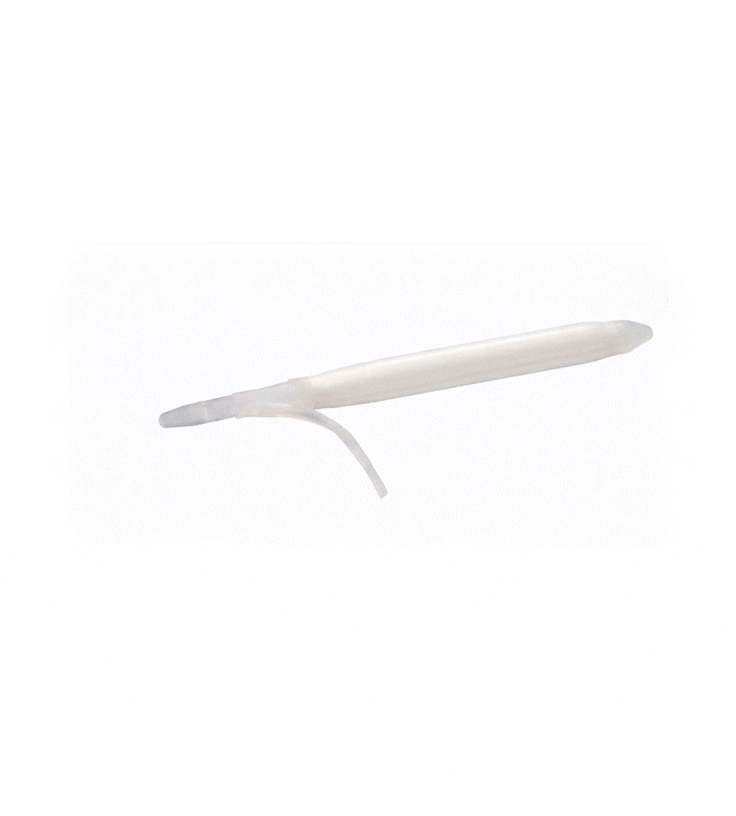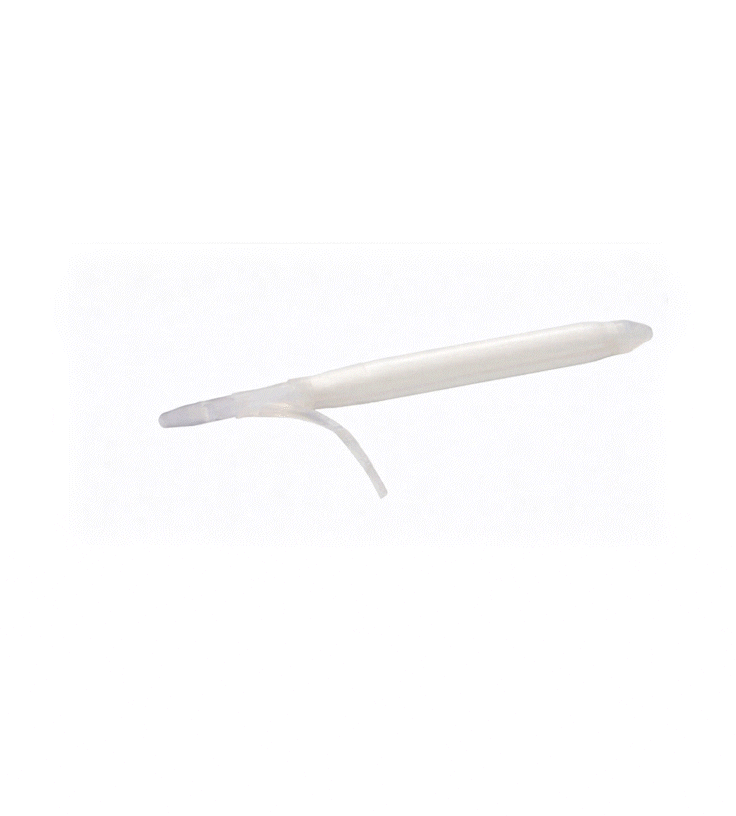
Kink-resistant tubes are connected easily and manually without the need for any additional assembly tools.
Kink-resistant tubes are connected easily and manually without the need for any additional assembly tools.


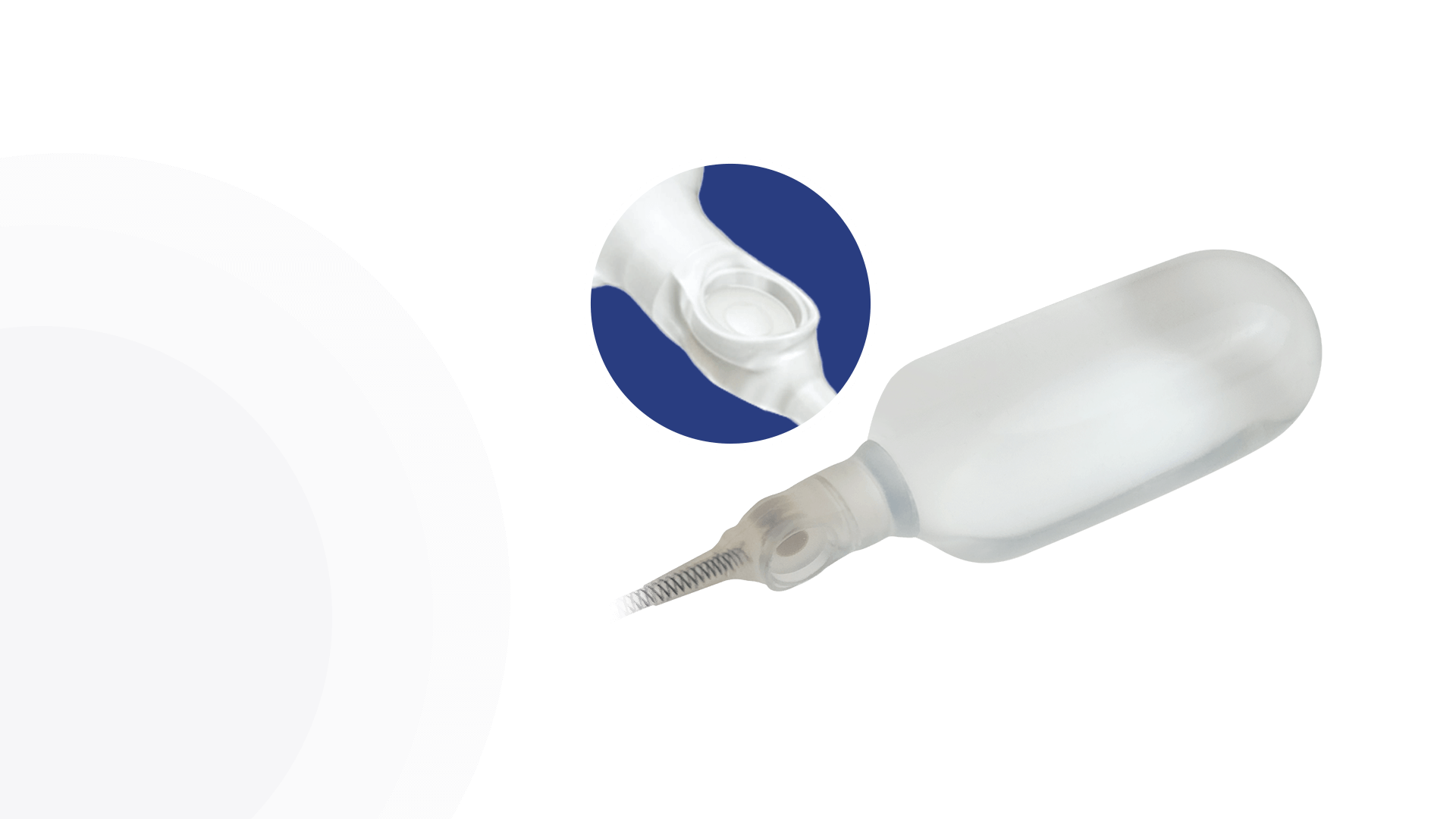
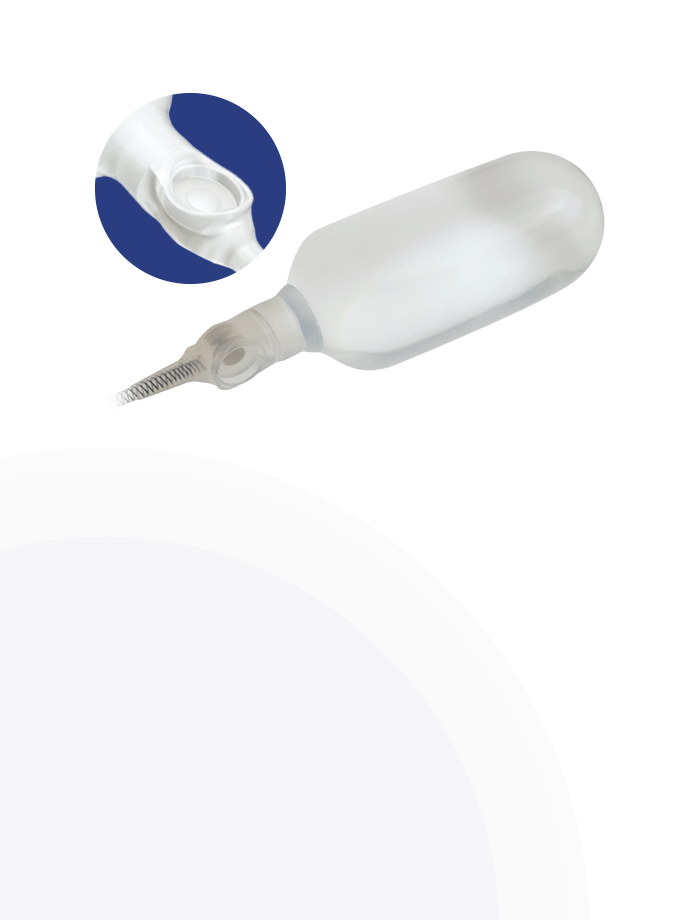
AdaptiveReservoir™

Pressure Valve
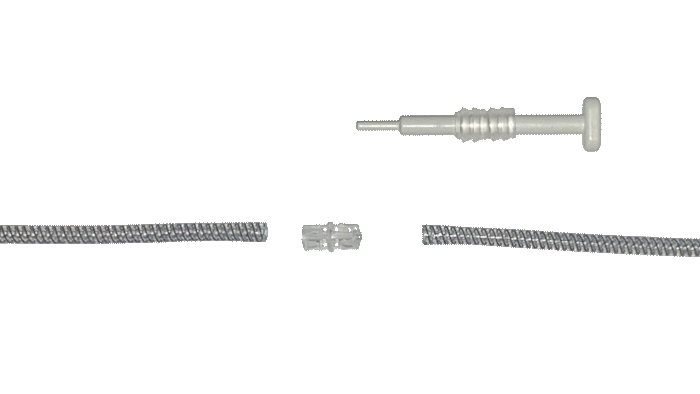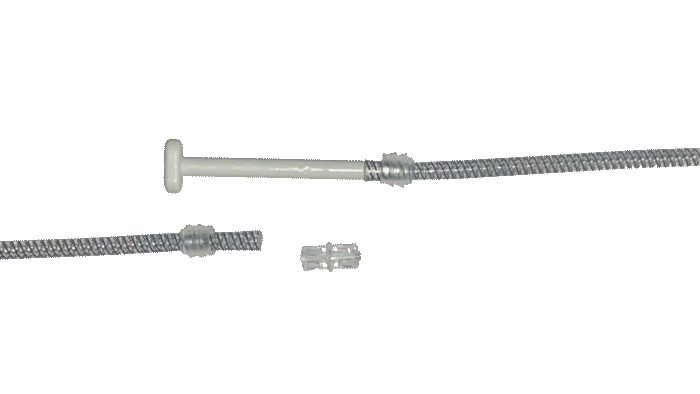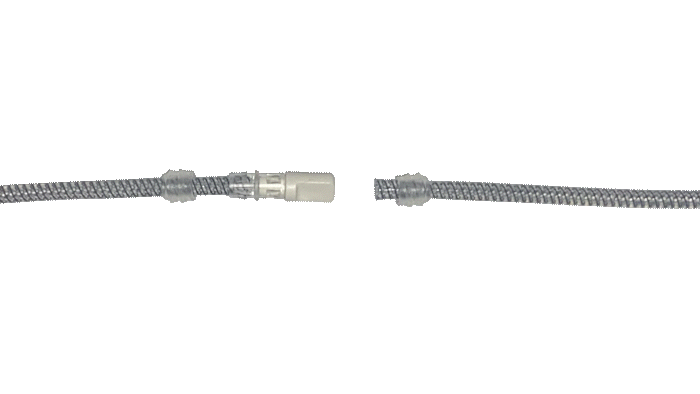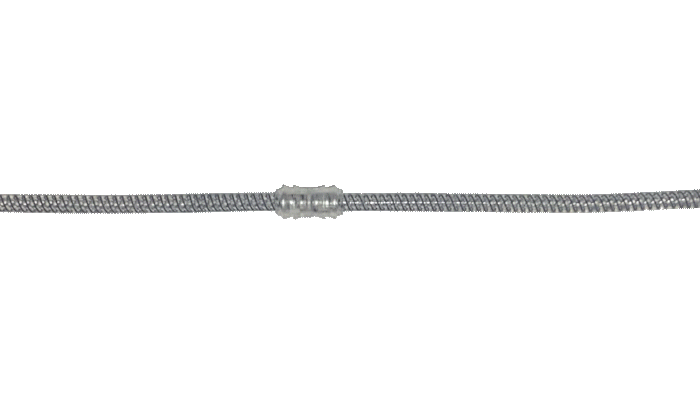
EasyClick™ Connector

Kink Resistant Tubing

Pulse™ Enhanced Deflation Button

Pulse™
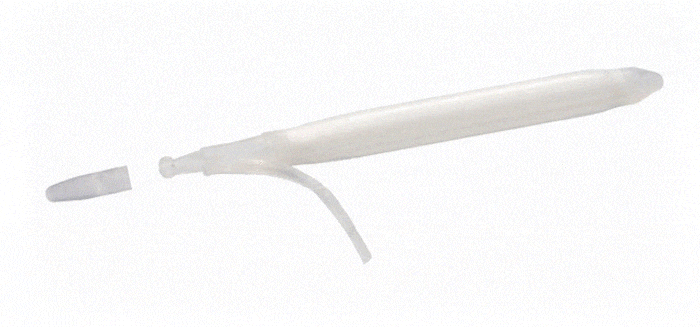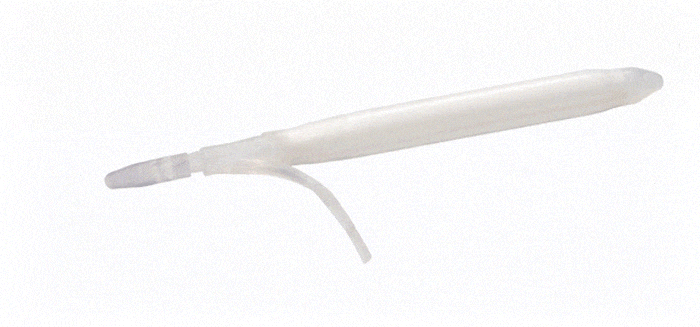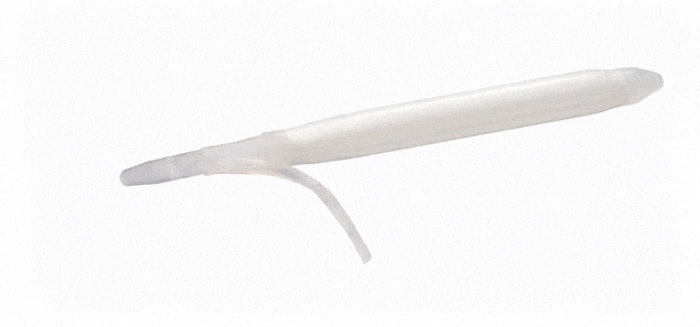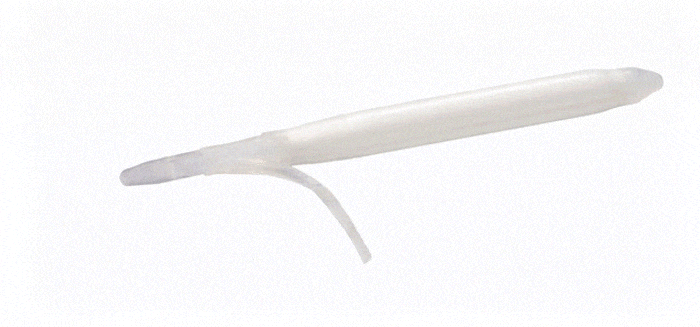
ConnectSecure™ RTE
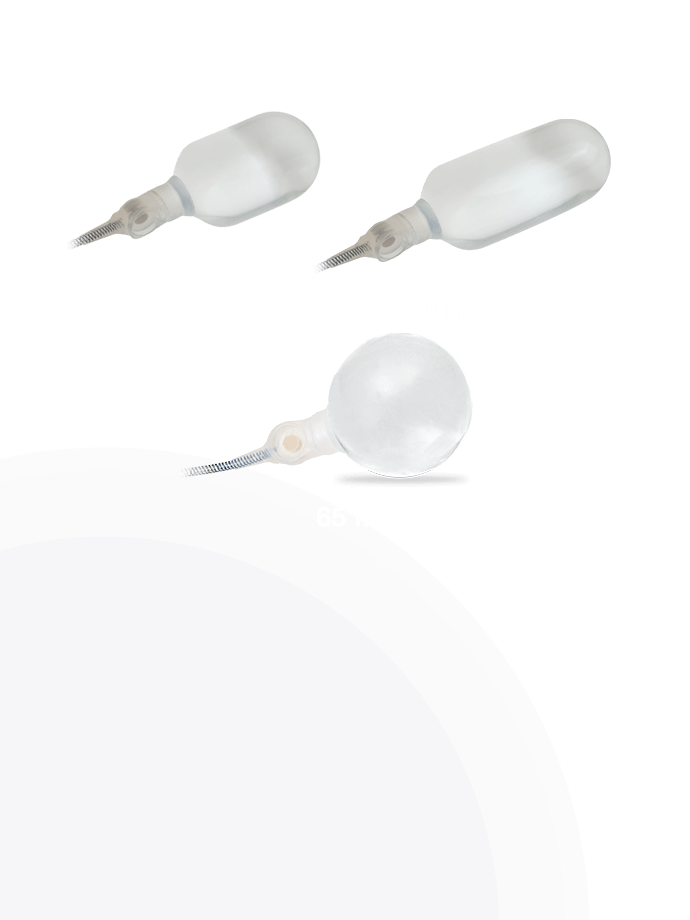
Additional 4th Layer

Infla10® Cylinders
- Infla10® Pulse™ AX Animation
- Infla10® Pulse™ X Animation
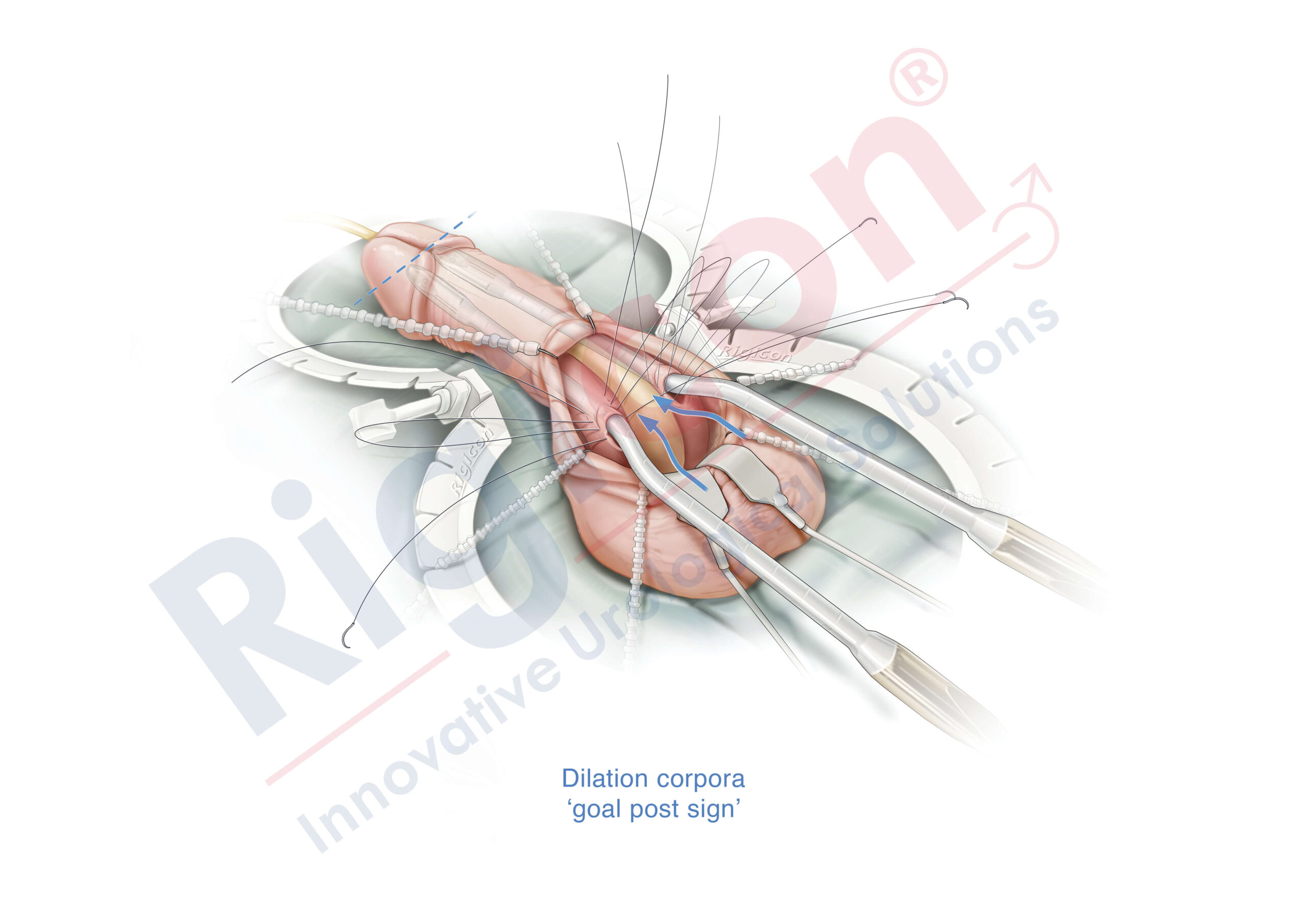
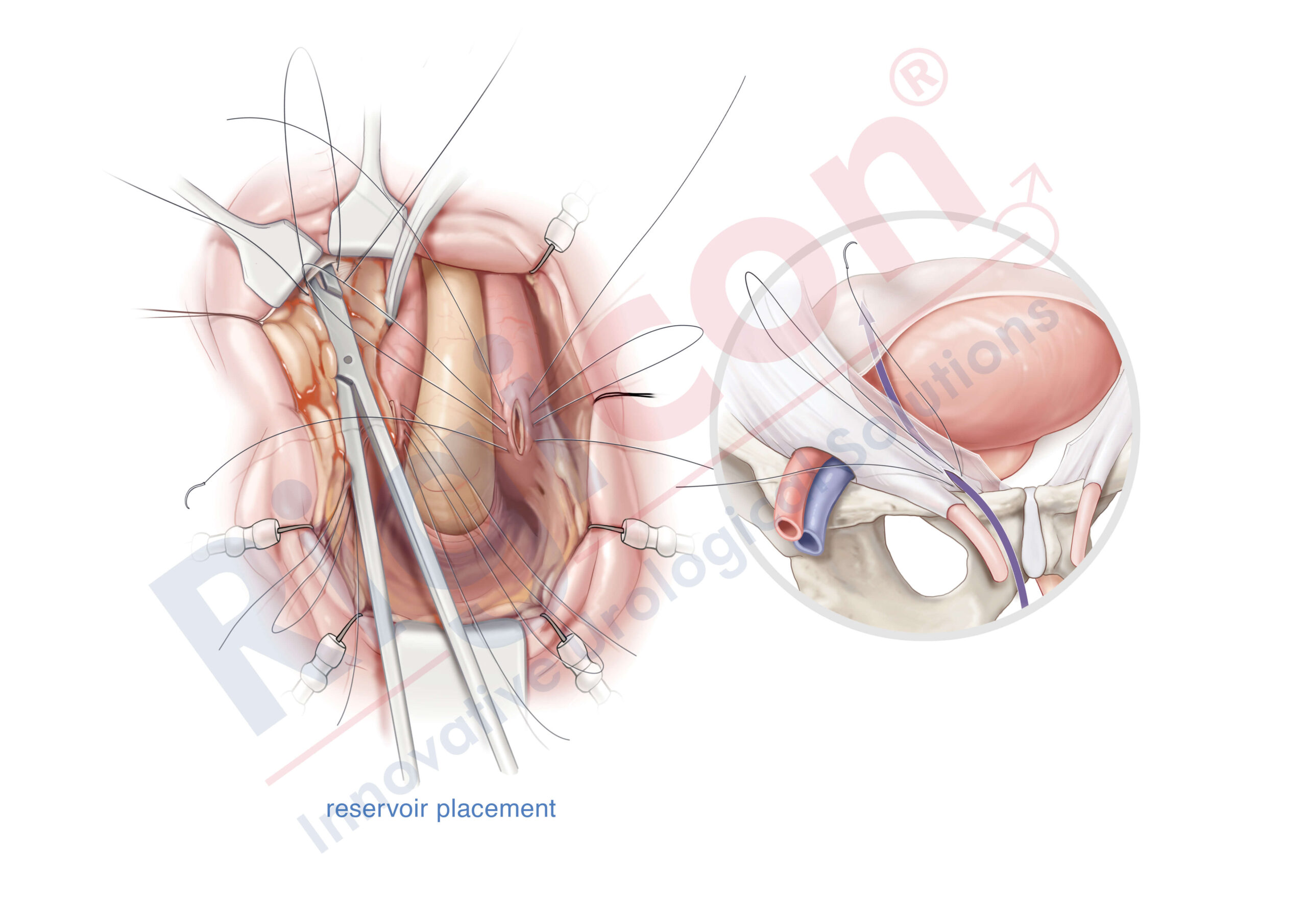
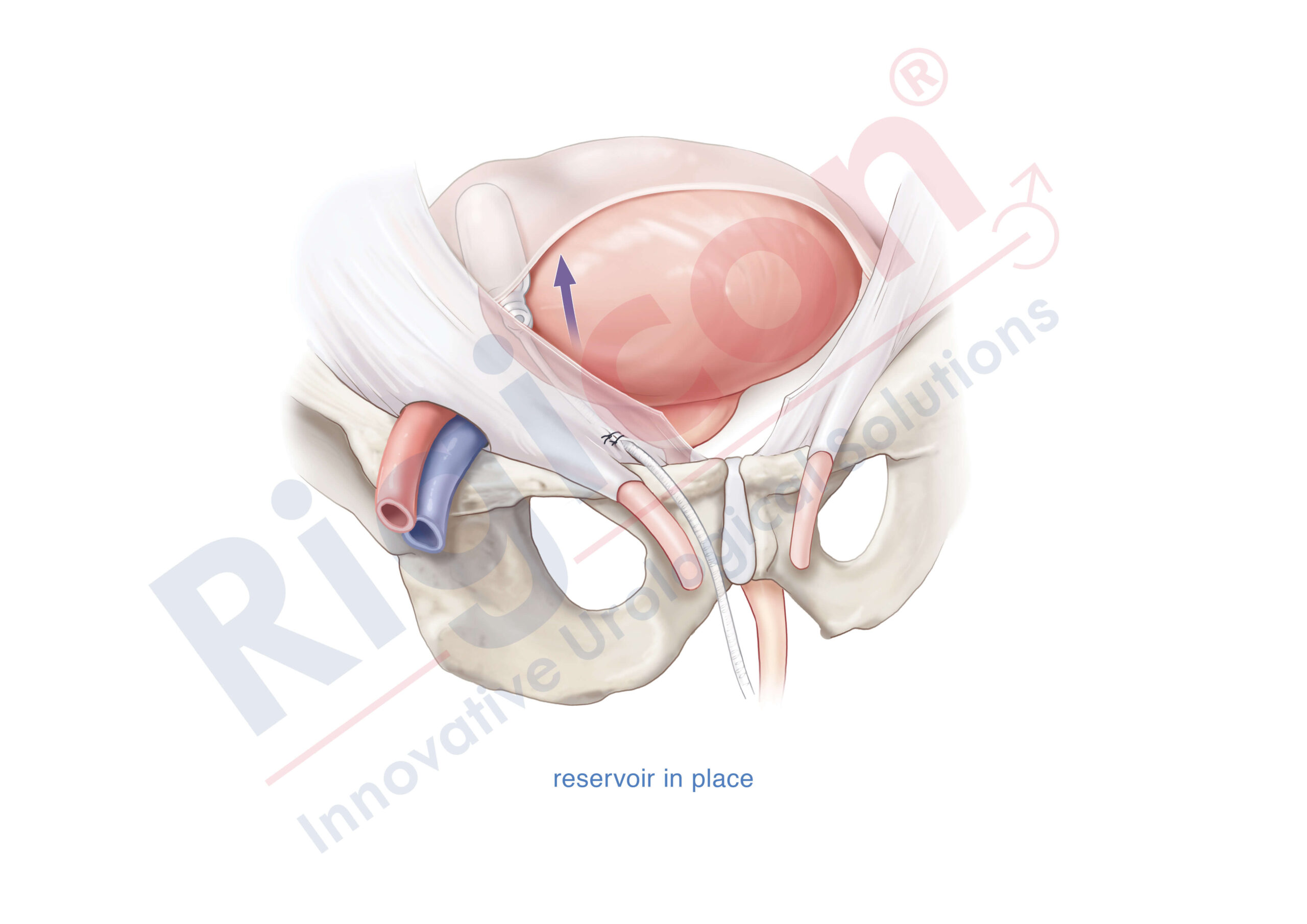
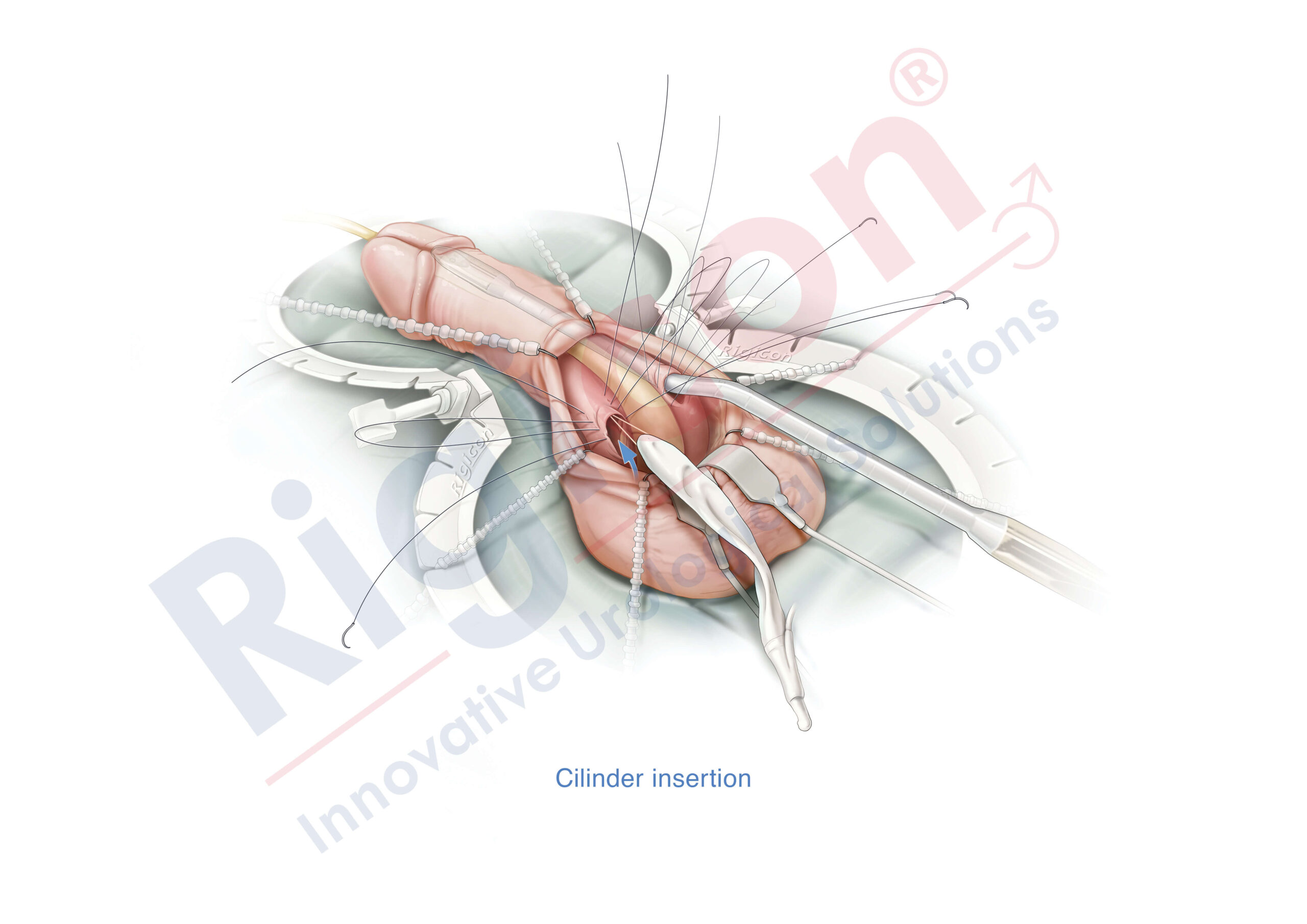
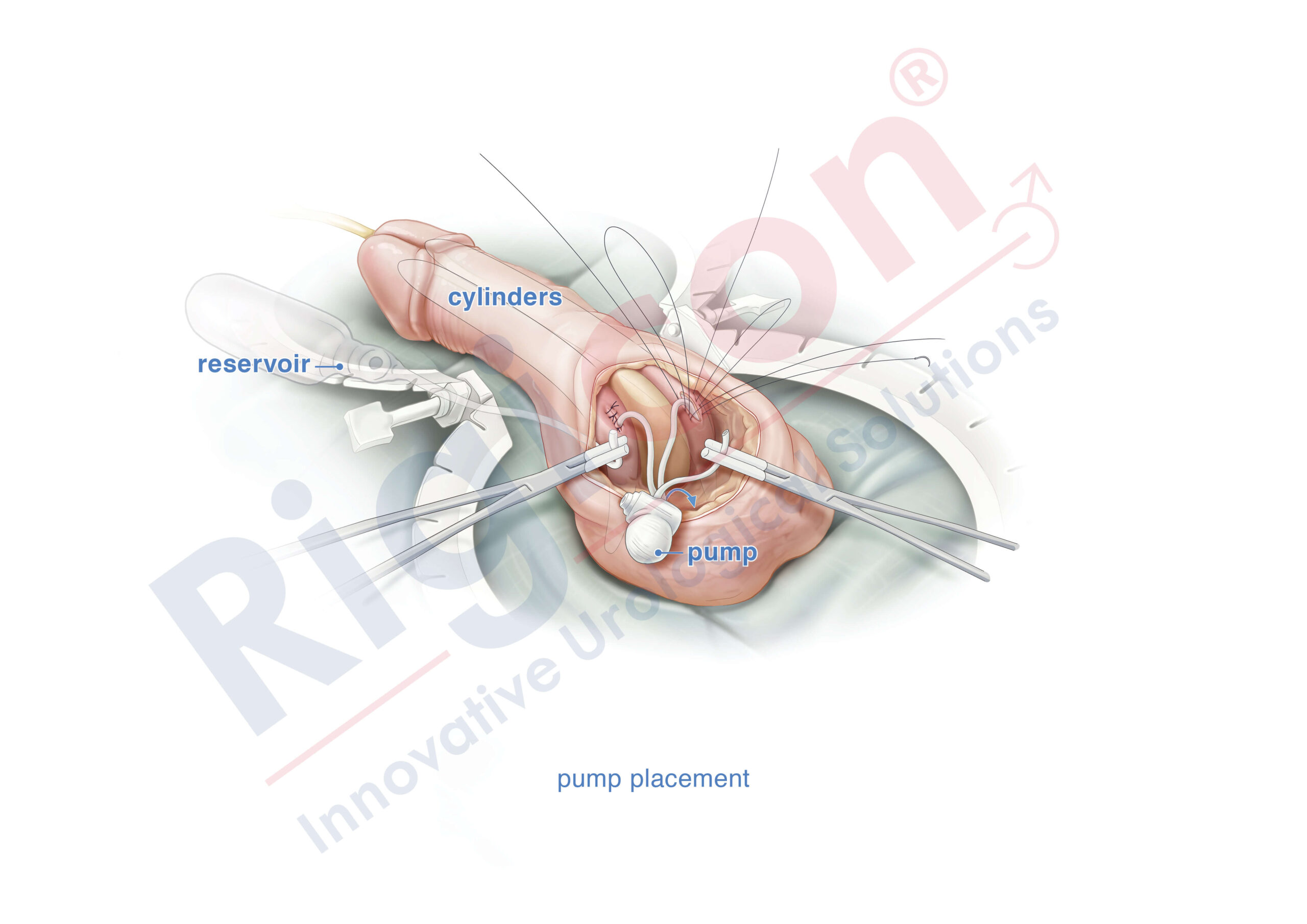
- Penoscrotal placement of the Infla10® Pulse™
- EasyClick™ Connector Assembly Instructions
- Infla10® Pulse™ AX Implantation [Case 1]
- Infla10® Pulse™ AX Implantation [Case 2]
- Unveiling Rigicon’s Infla10® Pulse™ AX: An Expert’s Perspective with Dr. Beley
- Infla10® Pulse™AX Patient Animation
- Rigicon Talks – Session 1 – Top 10 Questions Answered about Penile Implants
- Rigicon Talks – Session 3 – Myths and Facts About Penile Implants
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
These video resources are strictly for medical education. The footage contains graphic clinical procedures and is intended solely for healthcare practitioners.
Important: Video files are typically large. Download duration may fluctuate based on your internet connection speed. A robust, high-bandwidth connection is recommended for smooth playback and optimal learning experience.
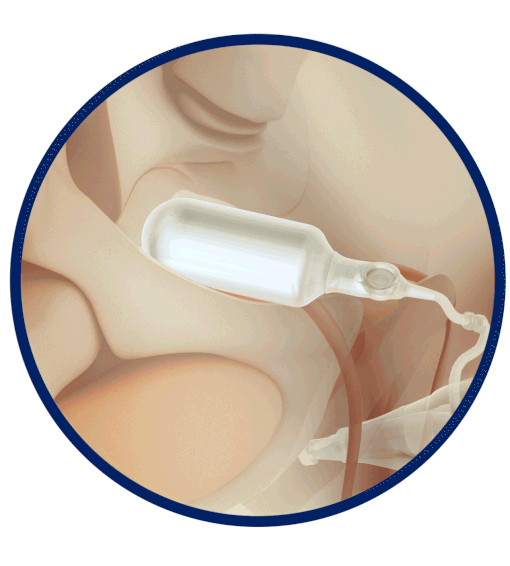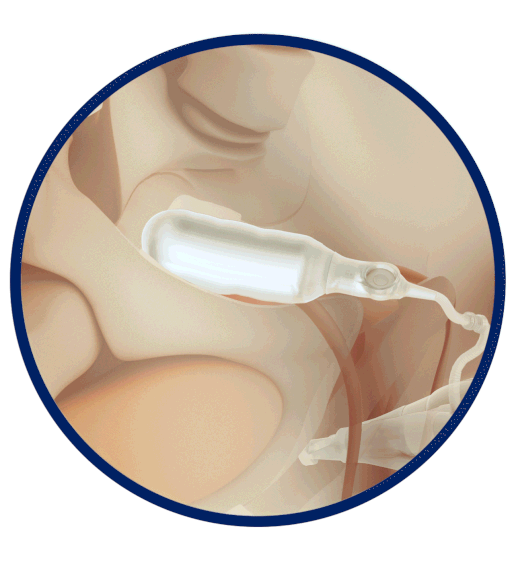
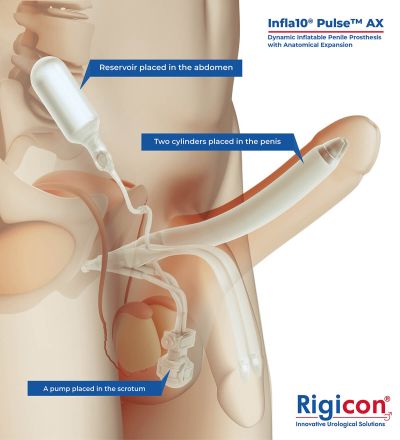
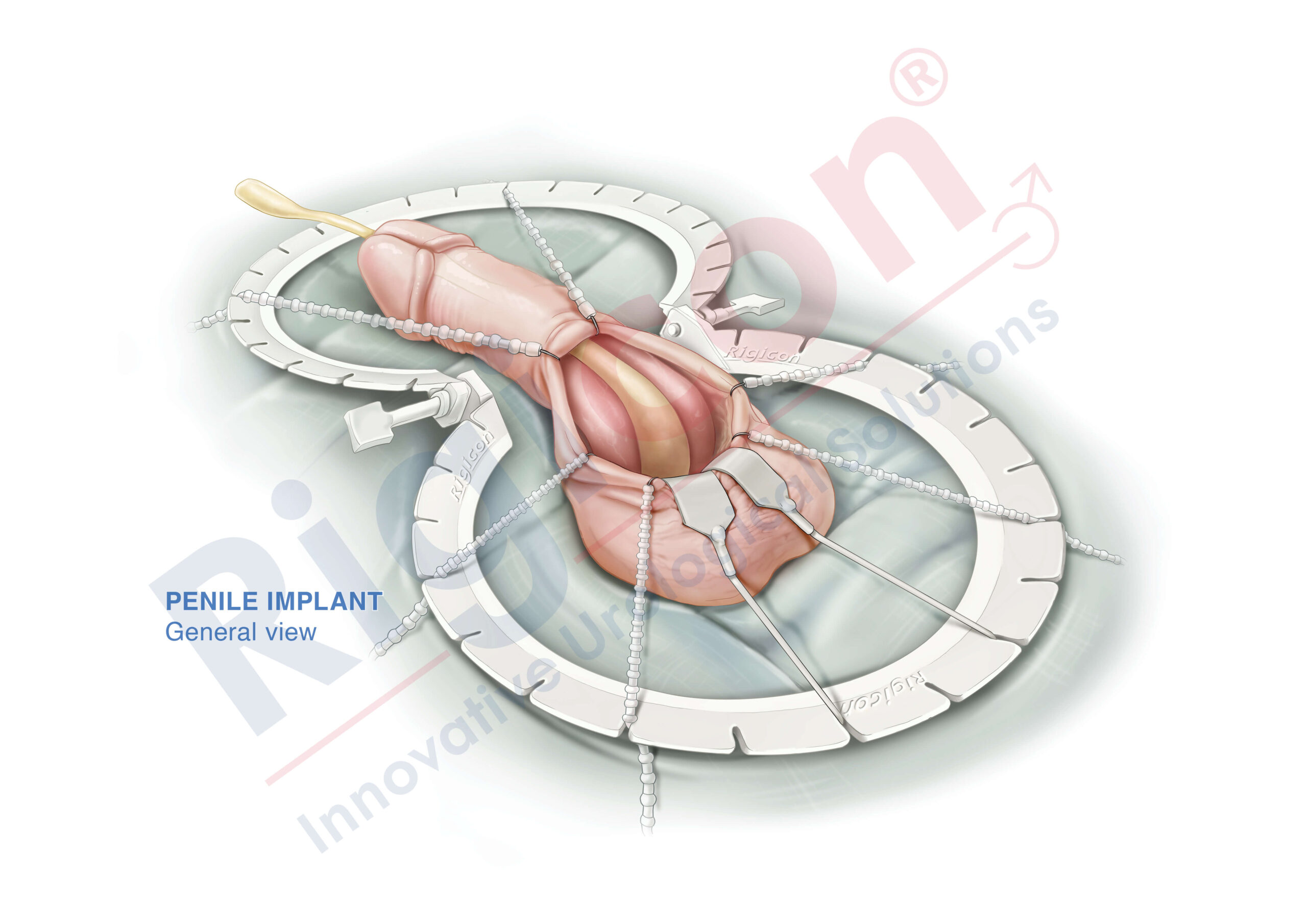
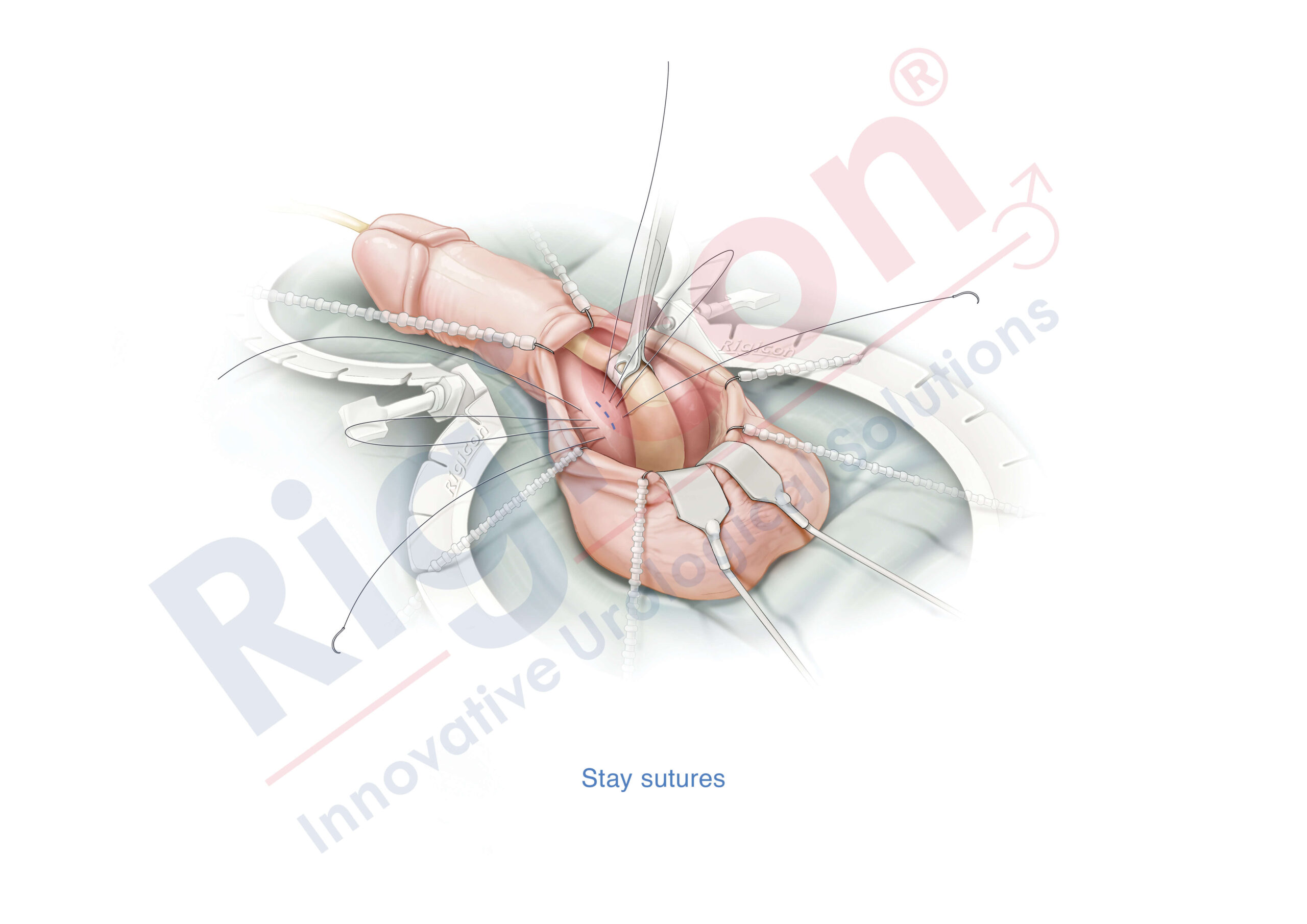
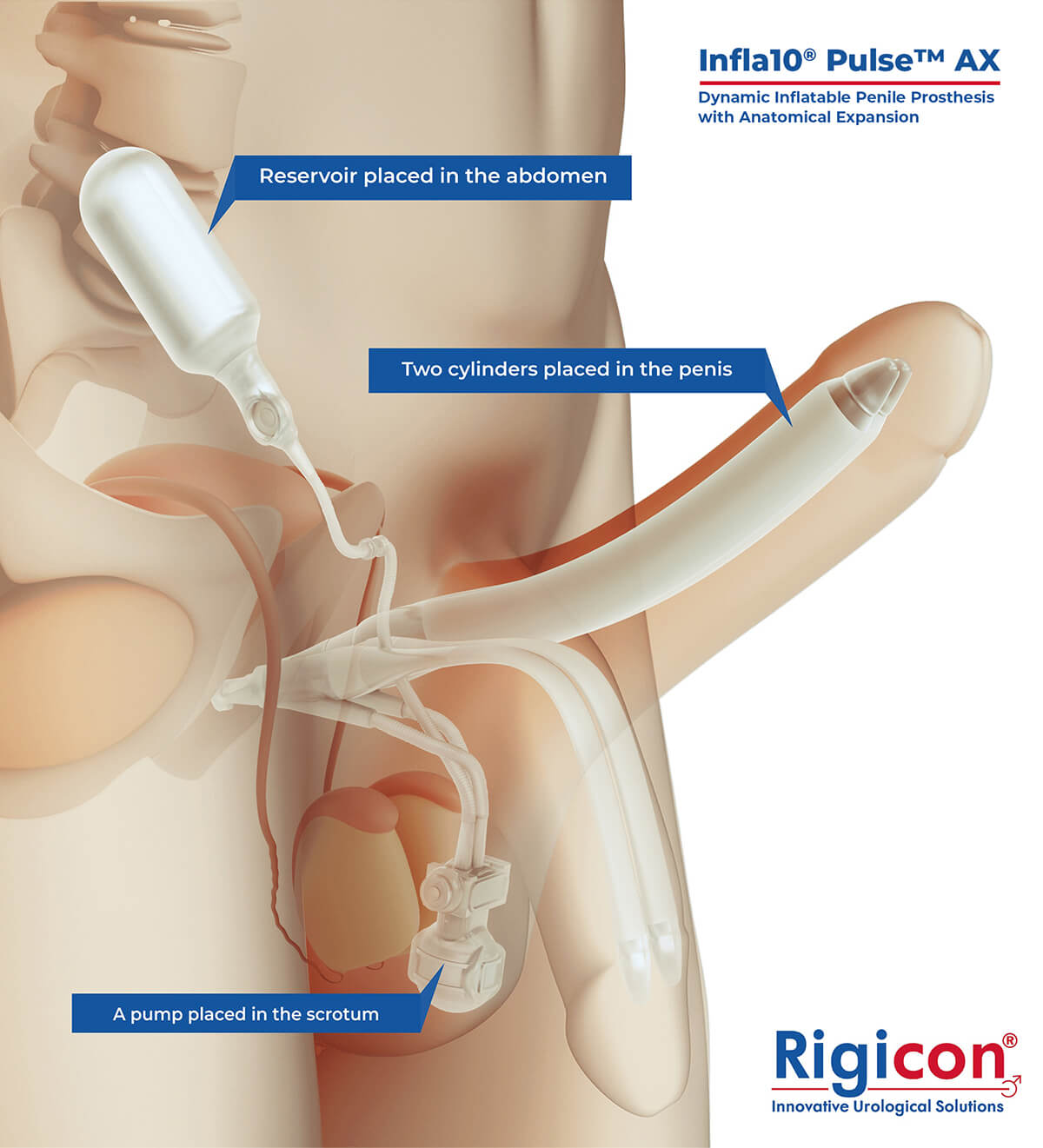
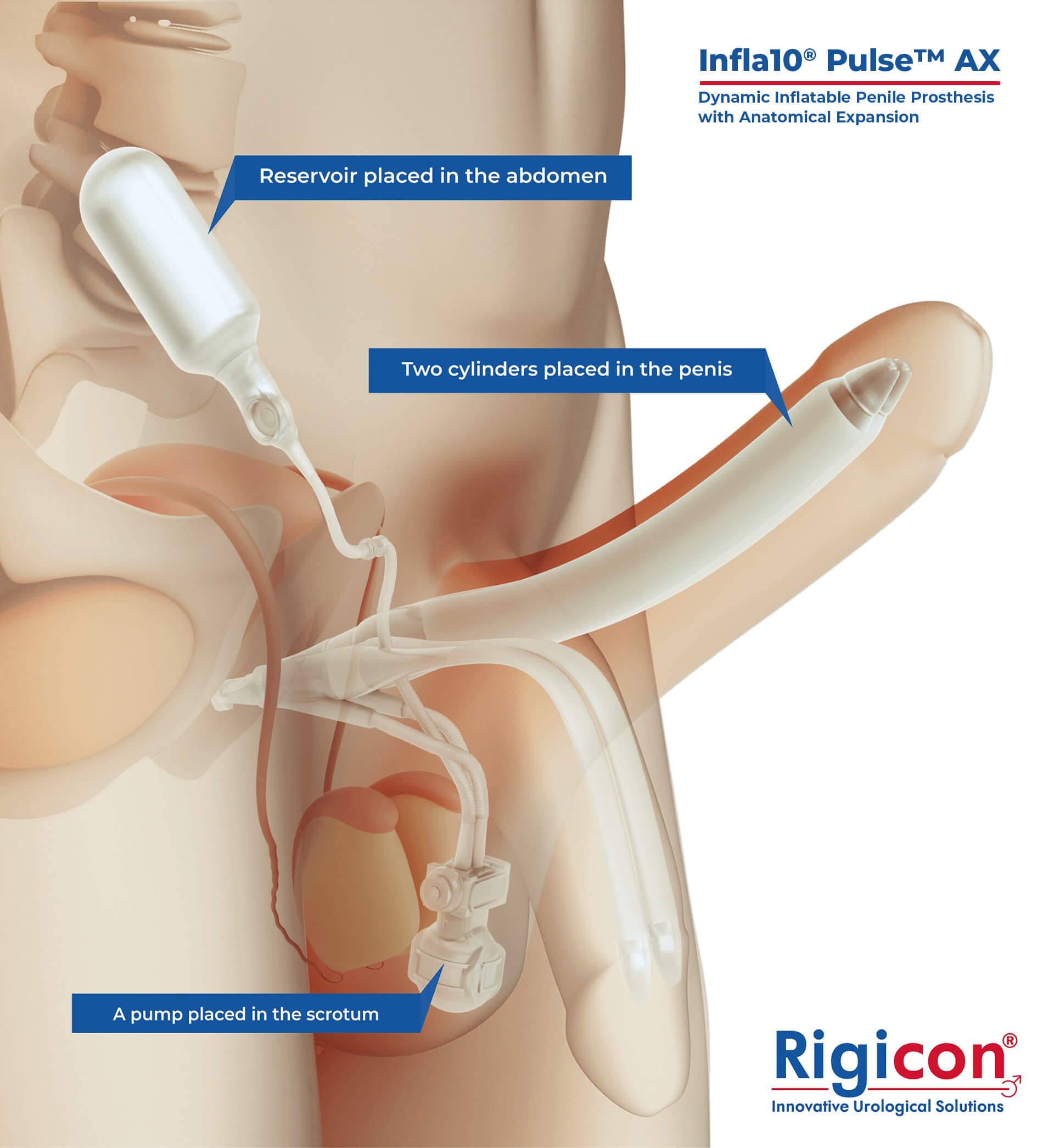
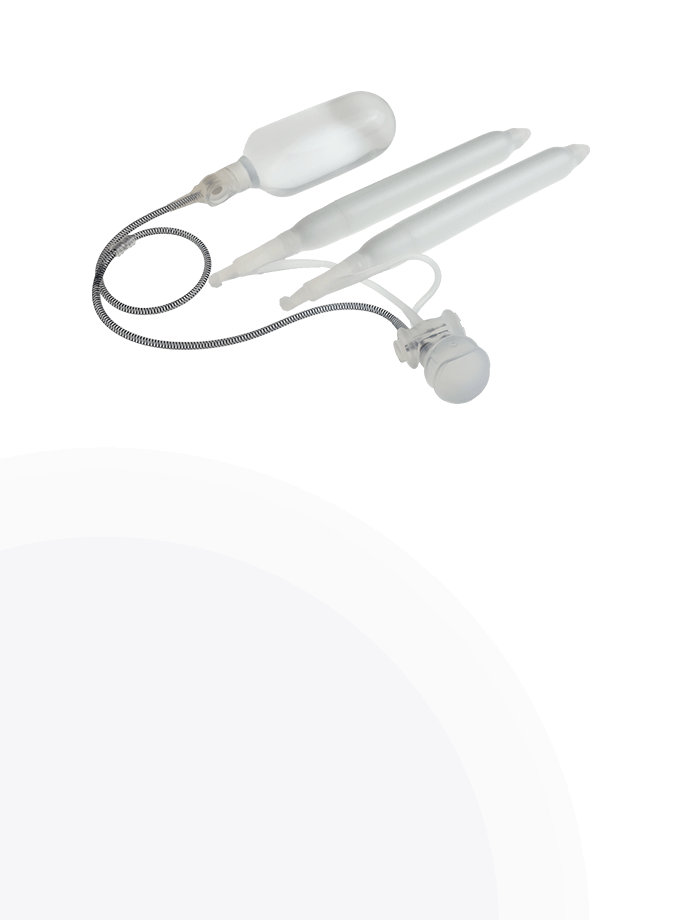
The Infla10® Pulse™ AX offers a potential option for men seeking a more natural erectile function with the convenience of an dynamic inflatable prosthesis. This three-piece system consists of two dynamic inflatable cylinders placed inside the penis, a fluid-filled reservoir placed in the abdomen, and a pump positioned discreetly in the scrotum. By squeezing the pump, fluid transfers from the reservoir to the cylinders, creating a firm and natural-looking erection.
The anatomical expansion feature aims to provide enhanced girth and a more lifelike experience, both when erect and at rest. This solution may be suitable for men desiring more control and satisfaction during intimate moments while maintaining a natural appearance.
The Infla10® Pulse™ AX offers a potential option for men seeking a more natural erectile function with the convenience of an dynamic inflatable prosthesis. This three-piece system consists of two dynamic inflatable cylinders placed inside the penis, a fluid-filled reservoir placed in the abdomen, and a pump positioned discreetly in the scrotum. By squeezing the pump, fluid transfers from the reservoir to the cylinders, creating a firm and natural-looking erection.
The anatomical expansion feature aims to provide enhanced girth and a more lifelike experience, both when erect and at rest. This solution may be suitable for men desiring more control and satisfaction during intimate moments while maintaining a natural appearance.
Product Code | Accessory Kit |
|---|---|
| INF10AK | Infla10® Accessory Kit |
What is erectile dysfunction (ED)?
It used to be called impotence. It is the inability to get an erection sufficient for sexual intercourse. It has nothing to do with sexual desire or libido. It has nothing to do with ejaculation of sperm, orgasm or feeling good. It is simply getting sufficient penile stiffness to have sex.
What is a penile prosthesis?
A penile prosthesis is a sterile device implanted into men to allow them to have sufficient penile rigidity to penetrate their sexual partner. There are two types:
- Malleable semi-rigid rods implanted through a tiny penile incision. A flexible wire allows the device to be straight for penetration or curved for concealment.
- Dynamic Inflatable Penile prosthesis which gives stiffness and increased girth by pumping a pump sending saline into the balloon like structures implanted into the two erectile chambers in the penis. The dynamic inflatable penile implant historically has been unitary, two-piece and three-piece. All models presently on the market are the three-piece because the erection and flaccidity are the best of the three types.
Is there any discomfort to activating an dynamic inflatable penile prosthesis?
Yes, at the beginning there will be soreness, like walking on a sprained ankle. With time however, the device activation is painless.
Can someone who views me without clothing know that I have a penile prosthesis?
No, the device is invisible in the genitalia.
How long will my erection last with a penile prosthesis?
Your erection is on demand and so is flaccidity. You can maintain the erection for as long as you like.
Will I have an orgasm with a penile prosthesis?
The penile implant has nothing to do with orgasm or ejaculation. Those are events that occur following sexual excitement. If you can orgasm and or ejaculate when suffering from ED, you will do likewise after the penile implant surgery.
Can I impregnate a woman with a penile prosthesis?
Yes, if the woman is fertile and you have not had a sterilization, sex with a penile implant can certainly result in pregnancy. In fact, many young men with diabetes seek surgical restoration of their ability to father a child by having penile implant surgery.
Can I have an imaging procedure called MRI with a penile implant?
Yes, all devices are MR Conditional which means you can be safely scanned. Special instructions are available from the manufacturer if you have a penile implant and need scanning.
What are the benefits of Rigicon Infla10® dynamic inflatable penile prosthesis?
The Infla10® appears to look like the competitive devices available worldwide. As the first new three-piece inflatable to appear on the market in decades, many subtle enhancements have been made after its evaluation by experienced implanting surgeons. The device is also coated with an infection retardant hydrophilic coating which allows your surgeon to add drugs to the aqueous solution to retard infection and decrease post-operative pain. Early studies published in the medical literature show the device is as safe or safer than its competitors that have been on the market for many years.
FAQ’s Disclaimer
The content is not intended to be a substitute for, nor does it replace professional medical advice, diagnosis, or treatment. If you have any concerns or questions about your health, you should always consult with a physician or other health-care professional.
-
Federal law (USA) restricts this device to sale by or on the order of a physician.
-
Not available for sale within the United States.